Species Table - Vapour Properties
Navigation: User Guide ➔ Species Table ➔ Vapour Properties
| Main | Editing User Species Database | Species Database Data Table - Theory and Equations | Viewing Properties | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species Database | Editing User Species Database | Importing data into Species Database | Species Table | Heat of Formation and Entropy | Density | Specific Heat (Cp) | Phase Change (solubility) | BPE & Acid/Base Ka/b | Vapour Properties | Transport Properties | Viewing Species Properties | Species Properties Reports |
Related Links: Vapour Liquid Equilibrium (VLE), Example for Vapour Pressure Data fitting
Introduction
These fields will only be writable for Vapour species, i.e. the user has chosen Gas for Phase Occurrence.
Vapour Pressure (Vp)
This field is optional.
If a component is to be used in Vapour Liquid Equilibrium (VLE) flash calculations, the Vapour Pressure must be provided for the vapour phase of the component. The Critical Temperature (Tc) must also be defined. The other critical constants and the acentricity value are also normally defined but may not be required, depending on the form of the equation chosen.
The user does NOT provide this information for steam, as SysCAD will use in-built equations to calculate the vapour pressure of water. Please see Water and Steam Properties.
Where the VLE flash calculations will be used (Single Component VLE or Multi-Component VLE), the user should also check that both the liquid and vapour phase of the component are defined and selected for the project. Where there are multiple liquid phase species for the component, currently only one of the liquid species will be used in VLE calculations.
The user may optionally specify a range of temperatures for the vapour pressure equation. Either or both of the limits may be specified.
Notes
- For all formats, the Critical Temperature (Tc) must be defined or the project will not load.
- For all formats, temperature T used in the equation is limited by the specified Critical Temperature (Tc). In other words if T>Tc, then Tc is used in the calculation.
- For all formats, temperature T used in the equation is limited by the specified Range Start value (if specified). In other words if T<Tstart, then Tstart is used in the calculation.
- The Range End value is only applicable to the Antoine format.
General format
| General Equation | SysCAD Format | Description |
|---|---|---|
|
One representation of the Vapour Pressure equation is: [math]\displaystyle{ \log_{10}(V_P) = \frac{a}{T} + b\log_{10}(T) + cT + d }[/math] |
This vapour pressure function can be used in one of these formats: | |
|
Where T is in Kelvin and Vp is in mmHg. SysCAD then converts the Vapour Pressure into kPa by multiplying 101.325/760 or 0.133322. | |
|
Where T is in Kelvin and Vp is in Atmospheres. SysCAD then converts the Vapour Pressure into kPa by multiplying 101.325. | |
|
Where T is in Kelvin and Vp is in KPa. | |
Prausnitz / Wagner Format
| General Equation | SysCAD Format | Description |
|---|---|---|
| Reid and Prausnitz (1987) have a large number of compound vapour pressures of the form (Equation 1) [math]\displaystyle{ \ln \left ( \frac{P}{P_c} \right ) = (1-x)^{-1} (Ax + Bx^{3/2} +Cx^3+Dx^6) }[/math] [math]\displaystyle{ x = 1-\left (\frac{T}{T_c} \right ) }[/math] |
|
Example Toluene: VpWagner36(-7.28067, 1.38091, -2.83433, -2.79168):Range(K, 309,) Benzene: VpWagner36(-6.98073, 1.33213, -2.62863, -3.33399):Range(K, 288,) |
|
The Fifth Edition (1993) uses a slightly different form [math]\displaystyle{ \ln \left ( \frac{P}{P_c} \right ) = (1-x)^{-1} (Ax + Bx^{3/2} +Cx^{5/2}+Dx^5) }[/math] |
|
See http://trc.nist.gov/TDE/Equations/FEquations.html Relies on the Critical Pressure (Pc) being specified. Projects will not load if Pc is not specified. |
Antoine format
| General Equation | SysCAD Format | Description |
|---|---|---|
| The Antoine Vapour Pressure equation is:
[math]\displaystyle{ \ln V_p = A - \frac B{T+C} }[/math] |
This vapour pressure function can be used in one of these formats: | |
|
where T is in Kelvin and Vp is in mmHg. SysCAD then converts the Vapour Pressure into kPa by multiplying 101.325/760 or 0.1333224. | |
|
where T is in Kelvin and Vp is in bar. SysCAD then converts the Vapour Pressure into kPa by multiplying 100. Also instead of ln Vp, uses log10 Vp. | |
Antoine format Discussion
The Antoine equation is generally intended for low pressures, up to approximately 2-3 bar (absolute). It is based on experimental data obtained at these relatively low pressures and is useful (and very accurate) in this range. It should not be used above the range indicated as it does not extrapolate well.
The Antoine equation has a lower limit as well, but for temperatures below this limit the material is typically solid; there is no downside to using the equation below the minimum value.
The Lee-Kesler equation is very accurate for high pressures, and is exact for the critical temperature, since the terms in the equation sum to unity at that temperature. It is also exact for [math]\displaystyle{ T_r = 0.7 T_c\, }[/math] by the definition of the acentric factor:
[math]\displaystyle{ w_{ac} = -\log \left(P_r^{sat}\right)_{T_r = 0.7} - 1\, }[/math]
It is less accurate for lower temperatures, especially for polar molecules. For example, for water it predicts a vapour pressure of 90kPa at 100°C compared to the true value of 1atm (101.3 kPa).
Therefore, SysCAD allows the user to specify a maximum temperature (Range End) for the Antoine equation. If this temperature is below the critical temperature, then SysCAD will use the Lee-Kesler equation (described below) instead of the Antoine equation from the maximum Antoine equation temperature until the critical temperature, if the Gas Constants Pc and Ac are available.
Note: If Pc and Ac are not available then the Range End value will be ignored and the Antoine equation will continue to be used up to the critical temperature (Tc).
See Transition from Antoine to Lee-Kesler for more information on how SysCAD handles the transition.
Lee-Kesler format
See Transition from Antoine to Lee-Kesler
| General Equation | SysCAD Format | Description |
|---|---|---|
|
The Lee-Kesler equation takes the form [math]\displaystyle{ V_p = P_c \; e^{L_0 \; + \; \omega_{ac} \; L_1} }[/math] [math]\displaystyle{ L_0 = 5.92714-\cfrac{6.09648}{T_r}-1.28862\ln(T_r)+0.169347 \,T_r^6 }[/math] [math]\displaystyle{ L_1 = 15.2518-\cfrac{15.6875}{T_r}-13.4721\ln(T_r)+0.43577 \,T_r^6 }[/math] |
|
Note:
|
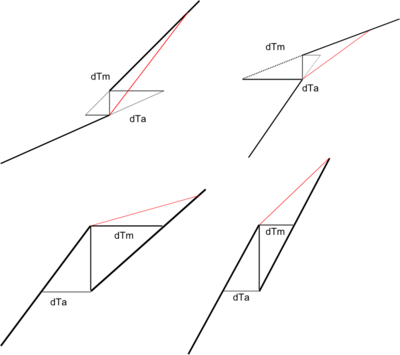
Transition from Antoine to Lee-Kesler
One concern with moving from the Antoine to Lee-Kesler equation is the transition between the two ranges. Discontinuities can lead to problems with the SysCAD solver, so SysCAD uses interpolation in the range just above the Antoine maximum limit (Range End).
To find the overlap range, consider the possibilities: the discontinuity may be positive or negative, and the slopes may not match.
SysCAD calculates [math]\displaystyle{ dT_a = \delta/f_{ant}'(t_{max})\, }[/math] and [math]\displaystyle{ dT_a = \delta/f_{mil}'(T_{max})\, }[/math], and (conservatively) sets the overlap range limit to be
[math]\displaystyle{ T_o = T_{max} + dT_a + dT_m\, }[/math]
Within this range SysCAD linearly interpolates between the two equations, so that if [math]\displaystyle{ \alpha = (T-T_{max})/(T_o-T_{max})\, }[/math] then
[math]\displaystyle{ V_p = (1-\alpha)f_{ANT}(T) + \alpha f_{LK}(T)\, }[/math]
Polynomial format
| General Equation | SysCAD Format | Description |
|---|---|---|
| A general polynomial equation for vapour pressure can also be used if required.
[math]\displaystyle{ V_p = a + bT + cT^2 + dT^3 + ...\, }[/math] |
|
Where a, b, c etc. are coefficients of the polynomial. Only 'a' is a required parameter, 'b', 'c' and 'd' are optional. Vp is in kPa and T is in Kelvin. |
Gas Constants
The multiple terms for gas data: Critical Constants, accentricity and boiling point (dew point) are defined by a single function
- For builds prior to Build 139, there are only the first four parameters
- Individual values are optional, so that if the critical volume is unknown then leave a blank between commas: Gc(190, 4.6, , .23, 290)
| SysCAD Format | Description of Terms |
|---|---|
|
Critical Temperature (Tc)This term is optional. The critical temperature is defined as the temperature above which the species will not condense, no matter what the pressure. This temperature is required in Kelvin. This is required for components involved in VLE flash calculations. This value is the maximum temperature that will be used in the vapour pressure equation. i.e. if [math]\displaystyle{ T \gt T_c }[/math], then [math]\displaystyle{ T_c }[/math] will be used in the vapour pressure equation. It is also required if the form of the vapour pressure equation chosen is the Antoine Equation with no parameters (i.e. VpAnt()). This is required for thermal conductivity if using Misic and Thodos Equation Formats. |
Critical Pressure (Pc)This term is optional. The critical pressure is the saturated pressure of the species at the critical temperature. Required in MPa. This is required if the form of the vapour pressure equation chosen is the Antoine Equation with no parameters (i.e. VpAnt()). This is required for thermal conductivity if using Misic and Thodos Equation Formats. | |
Critical Volume (Vc)This term is optional. The critical volume is the specific volume of the species at its critical temperature and pressure. Required in L/mol. | |
Acentricity (Ac)This term is optional. It is the Acentric Factor for the species.
where [math]\displaystyle{ T_r = \cfrac{T}{T_c} }[/math] is the reduced temperature, [math]\displaystyle{ p^{\rm{sat}}_r = \cfrac{p^{\rm{sat}}}{p_c} }[/math] is the reduced saturation vapour pressure. This is required if the form of the vapour pressure equation chosen is the Antoine Equation with no parameters (i.e. VpAnt()). | |
Normal Boiling Point (BP)Available from Build 139. This optional term is the Boiling point for the single species at standard pressure (1atm=101.325kPa) If supplied, along with a vapour pressure equation, will check for consistency. If not, the boiling point will be calculated from a supplied vapour pressure equation. |