Species Table - Thermodynamic Data
Navigation: User Guide ➔ Species Table ➔ Thermodynamic Data
| Main | Editing User Species Database | Species Database Data Table - Theory and Equations | Viewing Properties | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species Database | Editing User Species Database | Importing data into Species Database | Species Table | Heat of Formation and Entropy | Density | Specific Heat (Cp) | Phase Change (solubility) | BPE & Acid/Base Ka/b | Vapour Properties | Transport Properties | Viewing Species Properties | Species Properties Reports |
Introduction
This is information is generally required for all species, unless the project is not using the Energy Balance Add-On and/or with Heat Calculations switched off.
Notes:
- Species that have been added to represent gangue material, such as Inerts, Ore, etc. will generally not have any thermodynamic data.
- When importing Species from the HSC database that have a structural change at temperature, for example solids in smelters or furnaces where they change between alpha and beta states, SysCAD manipulates the Heat of Formation to take the enthalpy of phase change into account. Please see Importing Species from HSC Database.
Heat of Formation (H25)
This field is optional (but is usually defined).
This is the Heat of Formation at 25 °C. The unit is expressed in J/mol.
If this field is left blank, SysCAD will assume a value of 0 J/mol at 0°C.
For some Cp correlations, for example NASAGlenn_Cp and Gibbs_Cp, the value for H25 can be calculated directly from the Cp equation parameters. The user can then enter "FromCp()" which tells SysCAD to calculate the value for H25 for the enthalpy equation from the selected Cp equation. When selecting these types of Cp correlations and there are inconsistencies between the entered H25 and calculated H25 an error is given.
This value is used to calculate heats of reaction for chemical reactions specified in SysCAD. If the compound is used in chemical reactions, this number will determine the accuracy of the energy balance. If this field is left blank due to lack of data, then the user should define the Heat of Reaction (HOR) manually in the reaction file. See Reaction Block (RB) for further information on reactions.
Refer to Stream Heat of Formation values (Hf) for an example of how these individual heats of formation are used to determine the heat of formation of a stream.
Notes:
- All elements in their Standard State should have zero heat of formation at 25 °C.
- Examples of elements in their standard state: O2(g), N2(g), C(s) - where C is graphite.
- H+(aq) should have zero heat of formation at 25 °C. Projects with H+(aq) which use a non-zero value will not load.
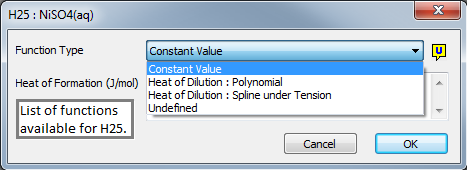
The image below shows the dialog box for the User values of H25:
- For most species, the user will enter a single Constant Value.
- However, in some cases, especially for acids or bases, the user may prefer to enter a range of H25 values for different mass fractions in solution. This allows SysCAD to then calculate the energy released or absorbed when solutions of different mass concentrations are mixed. This is discussed in the section below:
Heat of Dilution
This function is normally used for aqueous species, particularly for acid or basic species. The data entered for Heat of Dilution will enable SysCAD to calculate the amount of energy released (exothermic) or absorbed (endothermic) when two streams with different concentrations of the relevant species are mixed.
The most well known example of this effect is mixing concentrated sulfuric acid with water - which is extremely exothermic.
The heat of formation is a function of the mass fraction of the species in the solution (consisting of the species and the user specified solvent (usually water)). The user may enter the data:
- In the form of a polynomial equation, Heat of Dilution : Polynomial:
- Where the form of the equation is H25 = a + b*MF + c* MF^2 + d*MF^3 + e * MF^4
- MF is the Mass Fraction of the species in solution with the solvent, [math]\displaystyle{ MF = \cfrac{Solute}{Solvent + Solute} \, }[/math]; OR
- Where the form of the equation is H25 = a + b*MF + c* MF^2 + d*MF^3 + e * MF^4
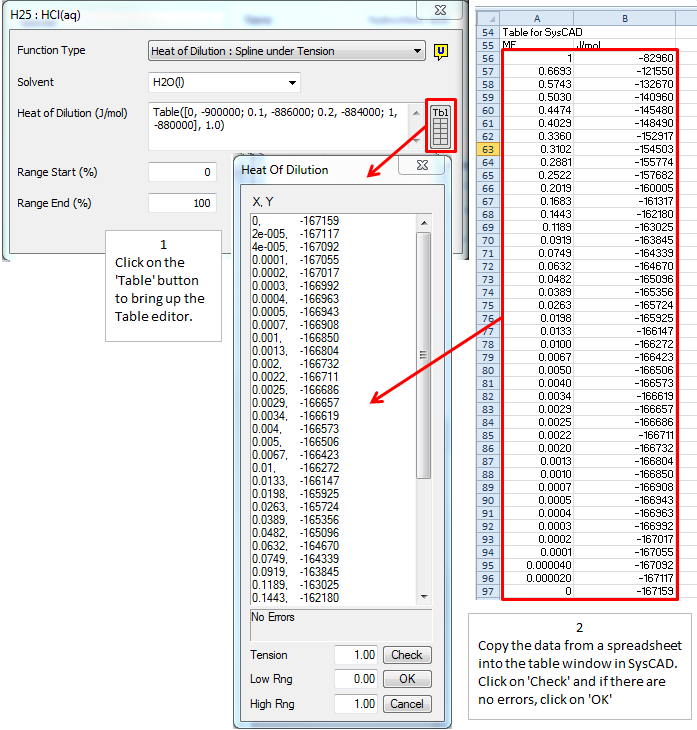
- As a table of data, Heat of Dilution : Spline under Tension:
- which will then use the TSpline method of interpolation. The data in the table is:
- X = Mass Fraction of species in solution with the solvent, [math]\displaystyle{ MF = \cfrac{Solute}{Solvent + Solute} \, }[/math], and
- Y = Heat of Formation at 25°C, in J/mol.
Notes:
- A polynomial will be processed more quickly within SysCAD and hence is preferred to a table of data.
- If you use a table of data (Spline under Tension), you must have data points for the mass fraction of the species at 0 and 100%.
- The best way to enter the table of values, is to have the data in an Excel spreadsheet in 2 adjacent columns - the first containing the Mass Fraction, and the second with the H25. Copy the 2 columns of data and paste them into the table in SysCAD. See the image below:
Heat of Transition
If the heat capacity range for a species does not include 25 °C (298.15K), then the enthalpy at the starting temperature of the heat capacity range (Ts) will be calculated as follows:
[math]\displaystyle{ H (Ts) = H_f (298.15K) + C_p \times (Ts - 298.15) }[/math]
where Cp is the heat capacity at Ts.
Thus if the enthalpy at Ts is known, the user can calculate what Hf should be entered in order to achieve this enthalpy in SysCAD.
This is similar to what SysCAD does automatically when importing species data from the HSC Database (refer to Importing Species from HSC Database).
Entropy (S25)
This field is optional.
This is the absolute entropy value for the species at reference temperature. The unit for Entropy is J/mol.K.
For some Cp correlations, for example NASAGlenn_Cp and Gibbs_Cp, the value for S25 can be calculated directly from the Cp equation parameters. The user can then enter "FromCp()" which tells SysCAD to calculate the value for S25 for the entropy equation from the selected Cp equation. When selecting these types of Cp correlations and there are inconsistencies between the entered S25 and calculated S25 an error is given. This is new to Build 138.
Entropy values are normally only used in Free Energy Minimisation (FEM) or adiabatic (isentropic) calculations. For isentropic calculation (e.g. compressor or turbine models), only differences in entropy are used, so this value need not be specified. However for FEM calculations, the absolute entropy is required and this value must be provided.
For gases (except steam), as well as integrating Cp/T with respect to temperature, a pressure term is included:
- -R * ln(P/Po)
- where R = gas constant
- P = partial pressure
- Po = reference pressure (1 atm)
Steam entropy is also a function of both temperature and pressure.
Refer to Stream Entropy (S) for an example of how these individual entropies are used to determine the entropy of a stream.
Note: H+(aq) should have zero entropy at 25 °C. Projects with H+(aq) which use a non-zero value will not load.