Species Table - Liquid Properties
Navigation: User Guide ➔ Species Table ➔ Liquid Properties
| Main | Editing User Species Database | Species Database Data Table - Theory and Equations | Viewing Properties | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species Database | Editing User Species Database | Importing data into Species Database | Species Table | Heat of Formation and Entropy | Density | Specific Heat (Cp) | Phase Change (solubility) | BPE & Acid/Base Ka/b | Vapour Properties | Transport Properties | Viewing Species Properties | Species Properties Reports |
Introduction
The two fields, Boiling Point Elevation (BPE) and the Disassociation constants (Ka/b), are only relevant for liquid species and hence they will not be visible for Solid or Gas species.
Both fields are optional, but will improve the quality of the solution results if they are completed.
Boiling Point Elevation
This field is optional.
The Boiling Point Elevation (BPE) is calculated for aqueous solutions using the following equation1:
- [math]\displaystyle{ BPE = K_b\sum m\times i \, }[/math]
where
- [math]\displaystyle{ K_b }[/math] is the Molal Boiling point elevation constant, or sometimes called the Ebullioscopic Constant.
- For water Kb = 0.512°C/m
- [math]\displaystyle{ m }[/math] is the molality of each aqueous species - moles/kg water.
- [math]\displaystyle{ i }[/math] is the van't Hoff constant for each aqueous species.
Notes:
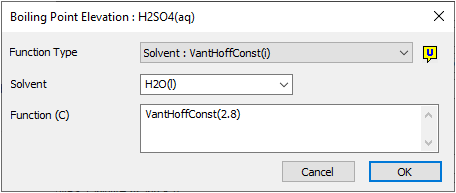
- The user may enter a van't Hoff Constant for each aqueous species in the Species Database. SysCAD will then use these values, together with the above equation to calculate the BPE for each stream when using the Standard Species Model and if van't Hoff is selected in PlantModel as the BPE Method in a project.
- SysCAD will provide a warning if the BPE calculation is not within some predefined range. The default range is between 0 to 20 °C. If the stream of interest has high concentrations, user may need to change the upper limit to remove the warning message. To change the limits, go to PlantModel - Species.
- The van't Hoff factor is a measure of the dissociation of the aqueous species in water. If the user cannot find a value for the van't Hoff factor in their references then the following approximations may be acceptable:
- For relatively soluble species, then the van't Hoff Factor = number of ions in the species, e.g. i(BeCl2) = 3, i(MnSO4) = 2
- For relatively insoluble species, then the van't Hoff Factor = 1, e.g. i(BaSO3) = 1
- The van't Hoff Factor is a function of the concentration of solution. As solutions become more concentrated, the potential for ion pairing increases. Thus, this is an approxiomation of actual behaviour.
See Boiling Point Elevation for a general discussion on this topic.
The BPE value may then be used in vapour liquid equilibrium calculations, please see Vapour Liquid Equilibrium (VLE) for more information.
Example 1 - Entering BPE data into the Species Database
Example 2 - Calculating BPE for the stream
For the following case:
Species |
Mass (kg) |
Moles (g moles) |
Molality |
van't Hoff Factor (i)
|
| Fe2[SO4]3(aq) | 10 | 25.01 | 0.025 | 4.4 |
| MgSO4(aq) | 15 | 124.6 | 0.125 | 1.21 |
| NaCl(aq) | 5 | 85.55 | 0.086 | 1.68 |
| H2O(l) | 1000 | 55508 | - | - |
- [math]\displaystyle{ BPE = 0.512*(0.025*4.4 + 0.125*1.21 + 0.086*1.68) }[/math]
- [math]\displaystyle{ BPE = 0.207 }[/math]°C
Reference
- Silderberg, M.S. “Chemistry - The Molecular Nature of Matter and Change”, 3rd Edition, 2003, pp508-509.
(Acid / Base) Dissociation (Ka/b)
This field is optional.
If the user requires a component to be included as an acid or base in the acidity, or pH, calculations, then this field must have the required acid or base dissociation constant. A number of 'standard' acids and bases are included in the default database with their associated dissociation constants. See Acidity Calculations for more information.
The acid/base dissociation constant is the equilibrium constant for the dissociation reaction of the particular acid or base in water:
For the equation [math]\displaystyle{ AB \rightleftharpoons A^+ + B^- }[/math], the dissociation constant, K (Ka for acid, Kb for bases), is calculated as:
- [math]\displaystyle{ K = \cfrac{[A ] [ B]}{[AB]} }[/math]
where [AB] = concentration of the acid/base in mol/L in water
The form of the variable is:
| ACIDS | BASES |
|---|---|
|
Ka(Ka1, Ka2, Ka3) where
Notes:
|
Kb(Kb1) where
Notes:
|
Examples: (with corresponding dissociation reactions)
| Species | Ka/Kb | NOTES | Dissociation Reactions |
|---|---|---|---|
| Hydrofluoric Acid (HF) | Ka(6.8e-4) | (a monoprotic acid) | [math]\displaystyle{ (HF \rightleftharpoons H^+ + F^- ) }[/math] |
| Phosphoric Acid (H3PO4) | Ka(7.2e-3, 6.3e-7, 4.2e-13) | (a triprotic acid) | [math]\displaystyle{ (H_3PO_4 \rightleftharpoons H^+ + H2PO4^-\rightleftharpoons H^+ + HPO4^{2-}\rightleftharpoons H^+ + PO4^{3-}) }[/math] |
| Sodium Hydroxide (NaOH) | Kb(1) | [math]\displaystyle{ (NaOH \rightleftharpoons Na^+ + OH^- ) }[/math] |