Sugar Species Model
Navigation: Models ➔ Sugar Models ➔ Sugar Species Model
| Sugar Properties | Sugar Unit Models | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sugar Species Model | Sugar Cane Shredder | Sugar Crusher | Sugar Crystalliser | Sugar Dryer | Sugar Fugal | Sugar Fugal 2 | Sugar Juice Screen | Sugar Mud Filter | Sugar Vacuum Pan |
Latest SysCAD Version: 25 February 2025 - SysCAD 9.3 Build 139.37016
Related Links: Species Models
General Description
The Sugar Species Models calculate thermo-physical properties of pure and technical sucrose solutions over the range of temperatures and concentrations found in the processing of sugar cane to sugar.
The correlations have been taken or adapted from public domain information. References and other relevant information are noted with the help for individual correlations. The calculated solution properties go smoothly to standard SysCAD water properties as solute concentrations go to zero. The correlations are self consistent and all functions are continuous and smooth over the entire range of applicability. The correlations are all based on Brix, Purity and Temperature where;
Brix The Brix includes all solutes in the liquid and is calculated as Brix = (total liquid mass - water mass). Any solutes may be included in the configuration file and used in the SysCAD model. Since the SysCAD sugar model only uses total liquid mass and water mass to calculate Brix, these will be implicitly included in the Brix and thus in the properties calculations.
Purity The purity is the mass of sucrose in solution divided by the total mass of solutes, Purity = Sucrose(aq)/Brix.
Configuring Sugar Species Model
In order for the Sugar Species Model to be available in a project, it must included in the configuration file of the project.
Insert into Configuration file
Sort either by DLL or Group.
| DLL: | Sugar.dll |
→ | Species Models | → | Sugar | |
| or | Group: | Sugar |
→ | Species Models | → | Sugar |
See Model Selection for more information on adding models to the configuration file.
Sugar Model Species
There is a set of species that have been developed for use with sugar solutions, although any species may be added. The species and descriptions are in the table below. There are some species which are comprised of pseudo elements which have been created in the SysCAD database.
| Sugar Species | ||
| Species | Formula | Description |
| Aqueous Species | ||
| Aqueous Sucrose | C22H22O11(aq) | Sucrose in solution |
| Reducing Sugars | C6H12O6(aq) | Generic formula for reducing sugars |
| Soluble Ash | SolAsh(aq) | Soluble ash - remaining mass after solution is pyrolized. Modeled as pseudo element Sa with a molecular weight of 180. |
| Soluble Protein | SolProt(aq) | Generic proteins in solution. Modeled as pseudo element Sp with a molecular weight of 180. |
| Soluble Other | SolOther(aq) | Any other unidentified solutes. Modeled as pseudo element So with a molecular weight of 180. |
| Solid Species | ||
| Crystalline Sucrose | C22H22O11(xt) | Crystalline sucrose |
| Amorphous Sucrose | C22H22O11(am) | Non-crystalline solid sucrose |
| Reducing Sugars | C6H12O6(s) | Solid generic reducing sugars |
| Soluble Ash | SolAsh(s) | Solid ash |
| Soluble Protein | SolProt(s) | Solid proteins |
| Soluble Other | SolOther(s) | Solid soluble other material |
| Fibre | C6H10O5(s) | Plant Fibre - modeled as Cellulose |
| Mud Solids | MudSolids(s) | Generic mud solids - composed of pseudo element inert solids, Is, with a molecular weight of 100. |
Notes on individual species used on the sugar model.
Soluble Ash, Protein and Other The stoichiometry of these species is not defined and they are simply modeled as pseudo elements. The properties calculations for sucrose solutions depend only on the concentration sucrose and the purity not on detailed chemistry of the impurities. This means that detailed composition of the impurities is not required to describe the solution.
Fibre Fibre in the species model is Dried fibre (no bound water - basically just cellulose). It is reported as "dried" fibre to coincide with the way that measurements on sugar cane and bagasse are physically done. Any bound water is implicitly included in the calculation of bagasse properties (i.e. solids fraction, Brix). This description is consistent with standard practice in the sugar industry.
The chemical formula and properties are assumed to be those of cellulose and the calculation of fibre Cp is derived from published data on Cp of plant fibre.
Mud Solids Mud solids are defined as generic inert solids. They do no react so a more detailed description is not required. However, in the models where there is any solids separation, the mud solids and handling of the mud solids actually includes mud and any other solids that are not fibre and there is no reason that other solids cannot be added into the system if required.
Sugar Model Properties
All of the properties have been adapted from the Sugar Technologists Manual (8th edition, Bubnik et al, 1995) with the exception of boiling point elevation which uses a more recent reference. The properties have been developed in a format that is compatible with iterative numerical calculations. Some of the original correlations only covered certain parts of the temperature and concentration ranges. Where different correlations were given for different ranges, the original functions were used to generate a matrix of data and a single new function fit over the whole range using two or three dimensional curve fitting techniques. In some cases, the original functions were modified slightly to match values and slopes the range boundaries. In all cases there is only one correlation spanning the range of data so that they are smooth continuous functions to help insure numerical stability for iterative calculations.
The solution properties default back to pure water properties as solute concentration goes to zero. The pure sucrose solution properties include corrections to give technical solution properties when the purity is less than 100%. The corrections are in functional forms that disappear as purity goes to 100%. This gives a continuous set of properties over the whole range of concentrations and purities with a single function for both pure and technical solutions.
The pure water properties are the IAPWS95 properties for water and steam that are built into SysCAD. The water properties used are calculated for saturation conditions at the given temperature.
NB Most of the correlations are valid for the processing of beet sugar although properties of very low purity solutions should be reviewed. Specific correlations for beet processing can be implemented on request.
| Property | Function | Description / Notes / Reference |
|---|---|---|
Crystalline Sucrose Density |
|
|
Amorphous Sucrose Density |
|
|
Sucrose Solution Density |
|
|
Sucrose Crystal Specific Heat |
|
|
Sucrose Crystal Enthalpy |
|
|
Sucrose Solution Specific Heat |
|
|
Sucrose Solution Enthalpy |
|
|
Solubility Pure Sucrose Solutions |
|
|
Solubility Technical Sucrose Solutions |
|
|
Sucrose solubility coefficient |
|
|
Sucrose Saturation Coefficient |
|
|
Sucrose Super Saturation |
|
|
Sucrose SuperSaturation Coefficient |
|
|
Non-Sucrose to Water Ratio |
|
|
Sucrose Solution Boiling Point Elevation |
|
|
Required Chemical Compounds
- H2O(l)
- C12H22O11(aq) - Sucrose in aqueous form
- C12H22O11(am) - Amorphous Sucrose in solid form
- C12H22O11(xt) - Crystalline Sucrose in solid form
References
Most of the properties have been taken adapted from the Sugar Technologists Manual (8th edition, Bubnik et al, 1995) with the exception of boiling point elevation which uses a more recent reference that is specific to CANE sugar solutions. A number of the solubility relations have been adapted from SRI work.
The solution properties have been developed so that they default back to pure water properties as sugar concentration goes to zero. The general approach was to use the Sugar Technologists Manual functions to create a matrix of data over the range of interest and then calculate the difference between pure water properties and the sugar solution properties to give a matrix of corrections to water properties as a functions of temperature, sucrose concentration and purity. The functional forms were chosen so that the correction goes to zero as the sugar concentration goes to zero. This way the properties of solutions are continuous over the whole range of concentrations including down to zero.
The pure water properties have been taken from the IAPWS95 work on water and steam properties. These are generally acknowledged as the most accurate set of properties available and match the water and steam properties built into SysCAD. The water properties used are calculated for saturation conditions at the given temperature.
The pure sucrose solution properties include corrections to give technical solution properties when the purity is less than 100%. The corrections for technical sucrose solutions are in functional forms that disappear as purity goes to 100%. This gives a continuous set of properties over the whole range of concentrations and purities and a single function may be called for both pure and technical solutions. NB If no purity is entered, the default purity is 100%.
Some of the original correlations only covered certain parts of the temperature and concentration ranges. Where different correlations were given for different ranges, the original functions were used to generate a matrix of data and a single new function fit over the whole range using two or three dimensional curve fitting software. In some cases, the original functions were modified slightly so that values and slopes matched at the range boundaries. In all cases there is only one correlation spanning the range of data so that they are smooth continuous functions to help insure numerical stability for iterative calculations.
Data Sections
Include Sugar Species Model in a Project
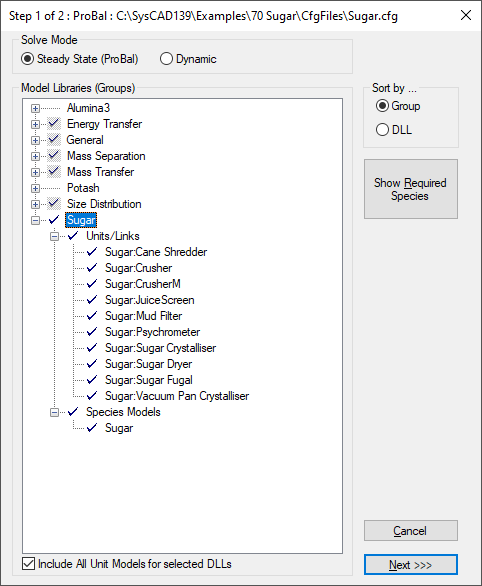
In Step 1 of Edit the configuration file, select the Sugar properties model from the list as show below:
In Step 2 of edit configuration file, make sure all the required compounds (under the Model Theory section) have been added to the species list.
Save the configuration file. Make a new project (or open an existing project) using this configuration file.
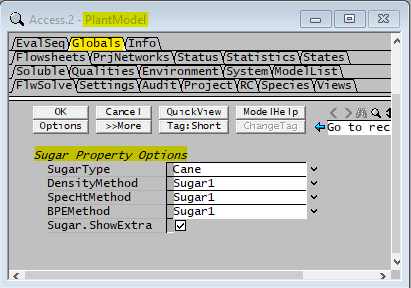
Selecting Global Property Calculation Methods
| Tag (Long/Short) | Input / Calc | Description/Calculated Variables / Options |
| Menu Command View - Plant Model - Globals Tab - Bayer Properties: | ||
| SugarType | Cane | The equations documented in the model theory section is based on sugar cane. |
| Beet | BEET sugar and Cane sugar syrups have different solubilities because of difference in the types of impurities. Corrections for BEET sugar are given in Sugar technologists Manual (pp 224, 342/1). | |
| DensityMethod | List Box | 1) Standard. 2) Sugar1 |
| SpecHMethod | List Box | 1) Standard. 2) Sugar1 |
| BPEMethod | List Box | 1) Standard. 2) Sugar1 |
| Sugar.ShowExtra | Tick Box | Selecting this will allow the user to view the Density, Cp and BPE calculated using the Sugar Properties model regardless of method chosen (above). Note these properties are for display purposes only, can be used to compare property calculation values between methods. |
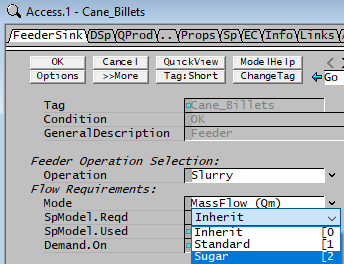
Selecting a Sugar Species Model in a Feeder
The user must have a project that includes a Sugar species model in the configuration file. It is then possible to select the Sugar model. On the first tab of the Feeder access window - 'SpModel.Reqd', choose Sugar or Inherit (if sugar is set as the default species model in the configuration file), as shown below:
| Tag (Long/Short) | Input / Calc | Description/Calculated Variables / Options |
| Model | Input | Inherit - Model used follows upstream units. |
| Standard - All the variables are calculated using the Mass Weighted Mean of the species. | ||
| Sugar - Density, Specific Heat, Enthalpy, Solubility, Boiling Point Elevation, etc. calculated using the equations defined for that sugar species model. |
Sugar Properties
NB These properties are for solutions only - these do not include any solid species in the calculations. For Bagasse where there is bound water associated with the fibre, the water must be taken into account in calculating properties such as Bagasse Brix (fibre is generally reported as dry fibre). For Massecuite where there is crystalline sucrose present, the general assumption is on the basis that is has been dissolved into solution. User calculations can be implemented in the configuration file Calculation_Configuration to display properties referenced to Massecuite and Bagasse .
| Tag (Long/Short) | Input / Calc | Description/Calculated Variables / Options | |
| --- Sugar Properties --- | |||
| SucroseMassFraction / Ws | Calc | Mass Fraction of Sucrose in Solution. | |
| Wds / BRIX | Calc | Mass fraction of total soluble solids in an aqueous sucrose solution or dry solids. | |
| Purity | Calc | The mass ratio of sucrose to the total soluble solids in an aqueous sugar solution. | |
| SucroseWaterRatio /SW | Calc | The mass ratio of sucrose to water in an aqueous sucrose solution. | |
| ImpurityWaterRatio / IW | Calc | The mass of non-sucrose solutes to water in an aqueous sucrose solution. | |
| BPE | Calc | Sucrose Solution Boiling Point Elevation | |
| --- Constant I/W Saturation Properties --- | |||
| SatPureSucroseWater / SWsatPure | Calc | See Solubility Pure Sucrose Solutions | |
| solubilitycoefficient / SC | Calc | See Sucrose solubility coefficient | |
| SatSucroseWater / SWsat | Calc | The mass ratio of sucrose to water in an aqueous sucrose solution at saturation for the given temperature and purity. | |
| SatImpurityWater / IWsat | Calc | The mass of non-sucrose solutes to water in an aqueous sucrose solution at the given temperature and purity. | |
| SucroseSaturation / WsSat | Calc | Sucrose Solubility - The mass fraction of a sucrose in a saturated solution at the given temperature and purity. | |
| SuperSatCoeff / SSNCoeff | Calc | The ratio of the mass of sucrose to water in an aqueous sucrose solution to that of a saturated solution at the given temperature and impurities to water ratio. See Sucrose SuperSaturation Coefficient. | |
| SucroseSuperSat / SSN | Calc | The mass ratio of sucrose in a solution to the mass of sucrose in a saturated solution at the same temperature and purity.[SuperSat=Ws/WsSat] | |
| --- Sugar Specific Properties --- (Visible only if the Sugar.ShowExtra tick box has been selected on View|PlantModel|Globals Tab. | |||
| Sugar.LRho | Display Only | Shows the Liquor Density at Temperature calculated using the Sugar Property Correlation. | |
| Sugar.LmsCp@T | Display Only | Shows the Liquor Specific Heat at Temperature calculated using the Sugar Property Correlation. | |
| Sugar.LmsHs@T | Display Only | Shows the Liquor Enthalpy at Temperature calculated using the Sugar Property Correlation. | |
| Sugar.LmsBPE | Display Only | Shows the Liquor Boiling Point Elevation calculated using the Sugar Property Correlation. | |