Discussion: Estimation of Specific Heat for Mineral Species
Navigation: Product Blog ➔ Discussion Pages ➔ Estimation of Specific Heat for Mineral Species
Related Links: Specific Heat
Heat Capacity Estimation
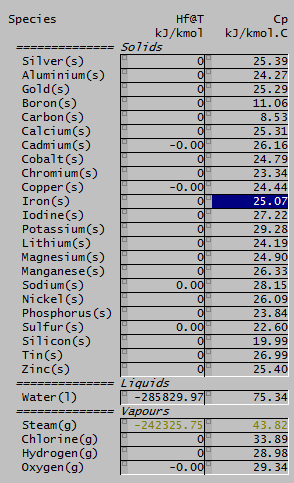
Here's a fun fact! The molar heat capacity for many solid elements is about 25 J/mol.K. For reference, you can see a SysCAD summary of common elements on the right.
In this discussion page we will look at why this is so, how we can use this property to check if specific heat values are reasonable and estimate the specific heat of minerals for which we have no data.
Dulong-Petit Law
The number 25 turns out to come from the value [math]\displaystyle{ 3\times R }[/math], the Universal Gas Constant (~8.3143 J/mol.K). This fundamental constant is available in PGM code (see Standard Constant Rgc). You have probably used [math]\displaystyle{ R }[/math] in an Arrhenius equation or in calculating the density of an ideal gas:
- [math]\displaystyle{ \frac\rho M = \frac p{RT} }[/math] where M is the molecular weight
That many elements share this value is the Dulong-Petit Law. Below are the molar heat capacities for all elements up to uranium[1]:
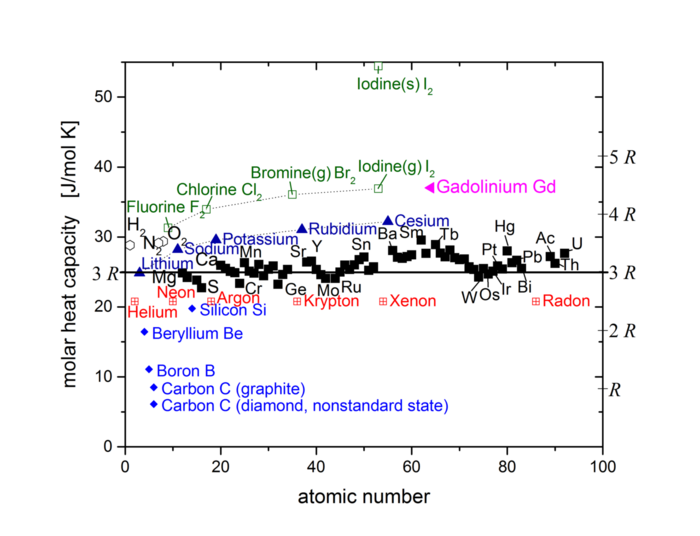
Apart from halogens (green), alkali metals (dark blue), noble gases (red) and a few other outliers, all elements cluster around the value 25 J/mol.K.
Kopp–Neumann Law
It turns out we can take this a lot further. The Kopp-Neumann Law (aka Neumann-Kopp Rule (NKR) or simply Kopp's law) states that we can estimate the molar heat capacity of a compound by adding the molar heat capacities of the individual elements (note that this applies mainly to solid species). For example, in modelling Bayer liquors and precipitation, we have to account for organic species, which are generally a mixture of formate/acetate/benzoate compounds. Rather than modelling each individual species, we often group these into a pseudo-organic species with a representative composition determined by analysis. The Bayer3 species model includes the psuedo-organic compound Na2C5O7(s), for which we can estimate the molar heat capacity to be 201.6 kJ/kmol.K, equivalent to a specific heat capacity of 0.92 kJ/kg.K.
- [math]\displaystyle{ C_{m,p}(Na_2C_5O_7) = 2 \times C_{m,p}(Na) + 5 \times C_{m,p}(C) + 3.5 \times C_{m,p}(O_2) }[/math]
- [math]\displaystyle{ c_p(Na_2C_5O_7) = \frac{C_{m,p}(Na_2C_5O_7)}{M} }[/math] where M is the molecular weight
By the same rule, the heat capacity of solid mixed oxides may also be estimated by adding the heat capacities of their component oxides.[2]
SysCAD Species Example
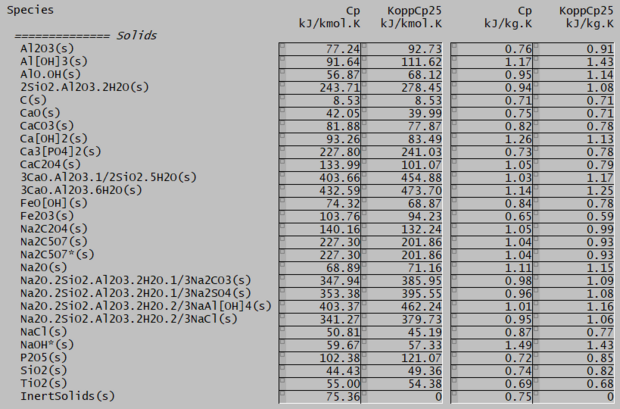
As an exercise, we have taken 500 solid species from the Default.93.db3 SysCAD database and calculated Cp(25) using both the relevant SysCAD Cp equation and Kopp's law, then sorted the results by relative difference. The full data table can be found below, but the smallest and largest errors are summarised here:
Cmp(kJ/kmol.K)
Species Kopp's Default Diff(%) Cp(kJ/kg.K)
--------------------------------------------------------------
Cu2Mg 73.8 73.8 0.013 0.49
CaO.MgO 79.5 79.6 0.044 0.83
MnSO4.5H2O 325.8 325.7 0.052 1.35
Cu6PS5I 310.7 310.5 0.056 0.44
NiSi2 66.1 66.2 0.137 0.58
CoO.Al2O3 132.0 132.2 0.174 0.75
.......
Fe2S3 117.9 47.8 59.468 0.57
Na2C2O4 132.0 34.0 74.248 0.99
2CaO.[Al2O3].8SiO2 567.1 97.7 82.781 0.82
CrO2 52.7 99.7 89.232 0.63
Ca3Al2Si3O12 360.5 1369.4 279.898 0.80
Ca2Al2SiO7 221.8 851.4 283.839 0.81
MgCO3.3H2O 208.4 -3689.2 1870.492 1.51 Cmp(kJ/kmol.K)
Species Kopp's Default Diff(%) Cp(kJ/kg.K)
--------------------------------------------------------------
Cu2Mg 73.8 73.8 0.013 0.49
CaO.MgO 79.5 79.6 0.044 0.83
MnSO4.5H2O 325.8 325.7 0.052 1.35
Cu6PS5I 310.7 310.5 0.056 0.44
NiSi2 66.1 66.2 0.137 0.58
CoO.Al2O3 132.0 132.2 0.174 0.75
CoS 47.4 47.5 0.189 0.52
Mg4Si6O21H12 701.5 702.9 0.210 1.14
CaMg3[CO3]4 310.1 309.4 0.231 0.88
Na8Fe2O7 378.0 379.0 0.256 0.93
FeSi 45.1 45.2 0.269 0.54
CaSO3.2H2O 179.2 178.7 0.306 1.15
Na2S2O3 145.5 146.0 0.347 0.92
CaCO3.MgCO3 155.3 155.9 0.403 0.84
MnSn2 80.3 80.0 0.437 0.27
FeO.SiO2 89.1 89.5 0.438 0.68
Ag2SO4 132.1 131.5 0.453 0.42
SnSO4 108.3 108.8 0.486 0.50
CaSO3 91.9 91.5 0.498 0.77
2CaO*SiO2 129.3 128.6 0.547 0.75
KFe3[AlSi3O10][OH]2 393.7 391.6 0.550 0.77
Mn3Si 99.0 99.6 0.608 0.51
Ag2O 65.5 65.9 0.628 0.28
SnS 49.6 49.3 0.663 0.33
Mg5[OH]2[CO3]4.4H2O 567.5 571.3 0.666 1.21
Fe2B 61.2 60.8 0.669 0.50
Ca2B2O5 146.1 147.1 0.687 0.80
AgP2 73.1 72.6 0.707 0.43
Na2S2O3.5H2O 363.7 361.0 0.749 1.47
CoSi2 64.8 65.3 0.751 0.56
CaMg2 75.1 75.7 0.764 0.85
MnSi 46.3 45.9 0.844 0.56
Al4Ca 122.4 121.3 0.851 0.83
ZnO 40.1 40.4 0.870 0.49
NiS2 71.3 70.6 0.919 0.58
Ca3B2O6 186.1 187.9 0.970 0.78
Na2S3 124.1 125.3 1.004 0.87
Fe2SiO4 128.8 130.2 1.053 0.63
Sn3S4 171.4 169.5 1.056 0.35
CoSn 51.8 51.2 1.090 0.29
CoSi 44.8 44.3 1.098 0.51
Mn2SiO4 131.3 129.9 1.104 0.65
CaS 47.9 47.4 1.114 0.66
Ag3PO4 158.7 156.9 1.124 0.38
MgO2 54.2 53.6 1.138 0.96
2CaO.2Al2O3.8SiO2.7H2O 965.2 954.0 1.163 1.05
NaOH* 57.3 58.0 1.205 1.43
NaOH 57.3 58.0 1.205 1.43
CuS 47.0 47.6 1.290 0.49
NiO.Al2O3 133.3 131.6 1.295 0.75
AuI 52.5 51.8 1.298 0.16
3CaO.Al2O3 212.5 209.7 1.308 0.79
Ni7Si13 442.5 436.7 1.310 0.57
3CaO.SiO2 169.3 171.6 1.349 0.74
Sn2S3 121.8 120.1 1.351 0.37
FeSi2 65.1 64.2 1.362 0.58
Fe2P 74.0 75.0 1.421 0.52
CuP2 72.1 71.1 1.431 0.57
CuFeO2 78.8 80.0 1.462 0.52
Cu2O 63.5 62.6 1.490 0.44
Mg2[OH]2CO3.3H2O 291.6 296.1 1.533 1.48
Mn5Si3 191.6 194.6 1.536 0.53
MgB4 69.1 70.2 1.539 1.02
Ni2Si 72.2 71.0 1.597 0.50
K2O 73.2 72.0 1.668 0.78
Al2Ca 73.8 72.6 1.684 0.79
CaZn2 76.1 74.8 1.702 0.45
3Na2O.2SiO2 311.6 306.2 1.717 1.02
MgCl2.6H2O 320.7 315.0 1.758 1.58
AlClO 55.9 56.9 1.829 0.71
AuSn 52.3 51.3 1.860 0.17
AuCu3 98.6 96.8 1.884 0.25
Fe3P 99.0 101.0 1.995 0.50
CaB2O4 106.1 104.0 1.996 0.84
AuCu 49.7 48.7 2.002 0.19
Na2CO3 108.8 111.0 2.017 1.03
MnS2 71.5 70.1 2.024 0.60
Au2P3 122.1 119.6 2.050 0.25
CuFeS2 94.7 96.6 2.053 0.52
MgB2 47.0 48.0 2.060 1.02
Mn3O4 137.7 140.5 2.076 0.60
MnS 48.9 49.9 2.080 0.56
Mn2O3 96.7 98.7 2.132 0.61
CaAl2Si4O12.4H2O 504.4 493.6 2.141 1.07
ZnSO4.H2O 150.3 153.6 2.155 0.84
FeSiO3 89.1 91.0 2.214 0.68
Ca3Si2O7 218.6 213.7 2.233 0.76
3CaO.2SiO2 218.6 213.7 2.259 0.76
Co2Al9 268.0 261.9 2.274 0.74
Ni3S4 168.7 164.8 2.278 0.55
3CaO.MgO.2SiO2 258.2 252.3 2.289 0.79
Na2SO3 122.9 120.1 2.291 0.98
ZnFe2O4 134.2 137.3 2.327 0.56
FeNaO2 82.6 84.5 2.379 0.74
Fe3Si 95.2 97.5 2.430 0.49
MgCO3 77.4 75.5 2.444 0.92
ZnSO4.2H2O 194.0 198.7 2.461 0.98
CaSi 45.3 46.4 2.463 0.66
FeO.Cr2O3 130.4 133.7 2.505 0.58
CoS2 70.0 68.2 2.534 0.57
MnO2 55.7 54.2 2.567 0.64
FeCr2O4 130.4 133.8 2.604 0.58
ZnO.2ZnSO4 253.4 246.6 2.685 0.63
Fe3Al2Si3O12 359.7 350.0 2.701 0.72
CoSO4 106.1 103.2 2.722 0.68
Co2B 60.6 59.0 2.747 0.47
ZnCO3 77.9 80.1 2.748 0.62
Na2CrO4 138.3 142.1 2.766 0.85
Mg2Si 69.8 67.8 2.800 0.91
Na2S2 101.5 98.6 2.827 0.92
NaS 50.7 49.3 2.828 0.92
2CaO.MgO.2SiO2 218.2 212.0 2.830 0.80
K2CO3 111.1 114.2 2.834 0.80
CaZn 50.7 49.3 2.835 0.48
SnS2 72.2 70.1 2.840 0.39
Ca3Fe2Si3O12 362.1 351.8 2.846 0.71
NiSi 46.1 44.8 2.852 0.53
ZnSO4.6H2O 368.5 358.0 2.871 1.37
SnI4 135.9 132.0 2.876 0.22
CdS 48.8 47.3 2.878 0.34
KMnO4 114.3 117.6 2.907 0.72
Al2S3 116.3 112.9 2.920 0.77
Na2O 71.0 68.9 2.923 1.14
2NiO.SiO2 130.8 127.0 2.926 0.62
Fe7Si8O22[OH]2 716.4 695.3 2.945 0.72
Cu2[OH]2CO3 159.7 164.5 2.961 0.72
CrO3 67.3 69.3 2.966 0.67
Zn3[PO4]2 241.2 234.1 2.976 0.62
Ca2Si 70.6 72.7 2.990 0.65
CoAl3 97.6 94.5 3.175 0.70
CuO.CuSO4 144.8 140.2 3.182 0.61
2FeO.SiO2 128.8 132.9 3.182 0.63
NiS 48.7 47.1 3.229 0.54
CaI2 79.8 77.2 3.252 0.27
CuI 51.7 53.4 3.292 0.27
Cu5FeS4 237.7 245.6 3.346 0.47
MgSO4.H2O 149.8 144.8 3.356 1.08
MgCl2.4H2O 233.4 241.2 3.374 1.40
MnCO3 78.9 81.5 3.388 0.69
CoB 35.9 34.6 3.396 0.51
2Na2O.SiO2 191.3 184.7 3.420 1.04
AuSn4 133.2 137.9 3.467 0.20
K2O.SiO2 122.6 118.3 3.507 0.79
CoCO3 77.3 80.1 3.536 0.65
NiP2 73.8 71.1 3.626 0.61
Al5Co2 170.9 164.7 3.633 0.68
SnI2 81.4 78.4 3.659 0.22
NaPO3 96.0 92.5 3.673 0.94
Na2S4 146.7 152.2 3.729 0.84
NaS2 73.3 76.1 3.729 0.84
MgB12 157.6 151.6 3.782 1.02
Mn2P 76.5 73.6 3.840 0.54
NaBO2 68.5 65.9 3.843 1.04
NiI2 80.5 77.4 3.884 0.26
Ag2S 73.4 76.2 3.892 0.30
Na2O.B2O3 137.1 131.7 3.924 1.04
CaSO4*2H2O 193.9 186.2 3.965 1.13
Ni3B 89.3 85.7 3.999 0.48
CoSO4.[6H2O] 367.9 353.0 4.060 1.40
Cu3[OH]2[CO3]2 236.7 246.6 4.175 0.69
Na4SiO4 191.3 183.2 4.205 1.04
Na2O2 85.6 89.2 4.206 1.10
FeSO4.7H2O 411.9 394.5 4.221 1.48
MgS 47.5 45.5 4.222 0.84
AlNi3 102.5 98.1 4.325 0.50
NiAl3 98.9 94.6 4.357 0.71
MnSiO3 90.3 86.4 4.359 0.69
Na2S 78.9 82.3 4.366 1.01
CaSi2 65.3 68.2 4.376 0.68
CaO.MgO.SiO2 128.9 123.2 4.390 0.82
2CoO.SiO2 128.3 133.9 4.439 0.61
CoI2 79.2 75.7 4.445 0.25
Ca5[PO4]3OH 403.2 385.1 4.502 0.80
Na2CO3.H2O 152.5 145.6 4.510 1.23
CaMgSiO4 128.9 123.1 4.512 0.82
CaSiO3 89.3 85.3 4.528 0.77
CaAl2Si4O12.2H2O 417.1 398.2 4.532 0.96
3Ca3[PO4]2.Ca[OH]2 806.5 769.9 4.543 0.80
Ni3S2 123.5 117.7 4.638 0.51
Ag2O2 80.1 83.8 4.641 0.32
Ca[OH]2 83.6 87.5 4.658 1.13
ZnS 48.0 45.8 4.667 0.49
AlB12 156.9 149.6 4.689 1.00
Zn2SiO4_gamma 129.5 123.3 4.733 0.58
MgNi2 77.1 73.4 4.755 0.54
Ni6P5 275.7 262.2 4.902 0.54
NiP3 97.6 102.5 4.971 0.64
Sn[SO4]2 189.5 180.0 5.027 0.61
CaO 40.0 42.0 5.044 0.71
CoP3 96.3 101.2 5.087 0.63
Ni2B 63.2 60.0 5.113 0.49
ZnSiO3 89.4 84.8 5.189 0.63
Cr8O21 494.7 520.6 5.235 0.66
CoSO4.[7H2O] 411.6 390.0 5.244 1.46
FeI2 79.5 83.7 5.246 0.26
CaFe[SiO3]2 178.4 168.9 5.329 0.72
Ni3Sn 105.3 99.6 5.372 0.36
AlCo 49.1 46.4 5.377 0.57
6CaO.6SiO2.H2O 579.5 548.3 5.383 0.81
MgSO4.6H2O 368.0 348.2 5.391 1.61
Ca3[PO4]2 241.0 227.8 5.461 0.78
FeSO4 106.3 100.4 5.576 0.70
SiS 42.6 45.0 5.601 0.71
Zn3P2 123.9 116.9 5.662 0.48
MgI2 79.3 74.8 5.663 0.29
NaI 55.4 52.2 5.668 0.37
CaC2O4.H2O 144.7 153.0 5.747 0.99
FeCO3 77.6 82.1 5.776 0.67
CuMg2 74.2 69.9 5.789 0.66
FeS 47.7 50.4 5.812 0.54
AuSn2 79.3 83.9 5.838 0.18
FeSi_alpha 45.1 47.7 5.856 0.54
AlB2 46.4 43.6 5.894 0.95
Zn2SiO4 129.5 121.8 5.902 0.58
Ca3P2 123.6 116.3 5.910 0.68
MgO 39.6 37.2 5.949 0.98
CaHPO4.2H2O 209.6 196.9 6.039 1.22
3CaO.[2SiO2].3H2O 349.5 328.4 6.053 1.02
Cu[OH]2 82.8 87.8 6.159 0.85
KHCO3 96.3 90.4 6.165 0.96
NaFe[SiO3]2 181.2 169.9 6.240 0.78
Mn3P 102.8 109.3 6.267 0.53
Cu6PS5Cl 300.4 319.4 6.336 0.49
K2O.2SiO2 171.9 160.9 6.361 0.80
CaSO4 106.6 99.6 6.506 0.78
AgIO3 96.6 102.9 6.536 0.34
KI 56.5 52.8 6.546 0.34
CuSO4 105.7 98.8 6.565 0.66
Ca2P2O7 201.0 187.8 6.576 0.79
FeAl2O4 132.3 123.5 6.598 0.76
CuI2 78.9 84.1 6.616 0.25
MnO.Al2O3 133.5 124.6 6.670 0.77
Mg[OH]2 83.2 77.6 6.687 1.43
MnI2 80.8 75.3 6.719 0.26
Cr5O12 292.7 312.5 6.750 0.65
NiB 37.1 34.6 6.762 0.53
Cu2O.Fe2O3 157.7 168.4 6.771 0.52
Cu2S 71.5 76.3 6.779 0.45
Ni4B3 137.5 128.1 6.835 0.51
Na2SO4 137.6 128.1 6.852 0.97
Mn3C 87.5 93.5 6.863 0.49
CoP 48.6 45.3 6.881 0.54
MnSO4 107.6 100.2 6.908 0.71
Fe5Si3 185.3 198.2 6.940 0.51
Ni2Al3 125.0 116.3 6.946 0.63
Ag2CrO4 132.8 142.1 7.047 0.40
Na2O.SiO2 120.3 111.8 7.069 0.99
Ca2Al2Si3O10[OH]2 364.1 338.2 7.126 0.88
ZnSO4 106.7 99.1 7.136 0.66
KClO4 104.9 112.4 7.155 0.76
CaCO3 77.8 83.5 7.230 0.78
AgP3 96.9 103.9 7.233 0.48
NaClO4 103.8 111.3 7.281 0.85
Co3O4 133.1 123.3 7.320 0.55
FePO4.2H2O 194.9 180.5 7.353 1.04
2CaO.Al2O3.SiO2 221.8 205.4 7.392 0.81
CaAl2Si2O7[OH]2.H2O 318.5 294.9 7.412 1.01
NaIO3 99.4 92.0 7.418 0.50
Fe2MgO4 133.7 143.8 7.514 0.67
MgSO4.7H2O 411.7 380.7 7.516 1.67
Na2O.2SiO2 169.6 156.8 7.543 0.93
Cu2SO4 130.1 120.3 7.578 0.58
SnO2 56.3 52.0 7.682 0.37
Ca2Sn 77.6 71.6 7.752 0.39
FeB 36.1 38.9 7.753 0.54
MnSO4.7H2O 413.1 380.7 7.835 1.49
Co2Si 69.6 75.0 7.849 0.48
FeO[OH] 68.9 74.3 7.879 0.78
Fe2NiO4 134.9 145.6 7.935 0.58
NaHCO3 95.2 87.6 7.944 1.13
CaSn 52.3 48.1 7.975 0.33
Na2B4O7 203.2 186.9 8.015 1.01
ZnSO4.7H2O 412.2 379.2 8.016 1.43
Ca2FeAl2Si3O12OH 389.4 358.1 8.021 0.81
Ag2CO3 103.3 111.6 8.036 0.37
Na6P2O8 333.9 306.7 8.159 1.02
CuO 39.1 42.3 8.171 0.49
AgI 52.6 56.9 8.186 0.22
Na4P2O7 262.9 241.3 8.243 0.99
CaB4O7 172.2 157.9 8.288 0.88
CuCO3 77.0 83.4 8.365 0.62
Ca2Al2SiO6[OH]2 265.5 243.2 8.389 0.91
Mg2SiO4 128.5 117.7 8.398 0.91
2CaO.5MgO.8SiO2.H2O 716.1 655.6 8.443 0.88
NaAlSi2O6.H2O 224.1 204.8 8.589 1.02
CaO*Al2O3*2SiO2 231.2 211.3 8.591 0.83
NaAlSi3O8_gamma 229.7 209.9 8.637 0.88
NiO 40.8 44.3 8.650 0.55
CaAl2SiO6 181.8 166.0 8.731 0.83
NiAl 50.4 46.0 8.734 0.59
Mg3Si2O5[OH]4 304.7 277.9 8.781 1.10
3CaO.Al2O3.6H2O 474.3 432.6 8.804 1.25
MnSO4.H2O 151.2 137.9 8.819 0.89
CaAl2Si2O8 231.2 210.7 8.867 0.83
CaO.Al2O3.SiO2 181.8 165.7 8.879 0.83
AlO 38.9 35.5 8.936 0.91
MgCl2.2H2O 146.1 159.2 8.986 1.11
Na2B8O13 335.4 304.9 9.107 0.99
Na2P2O6 192.0 174.5 9.125 0.94
MgSO4.2H2O 193.5 175.7 9.167 1.24
MnO 41.0 44.8 9.186 0.58
SiP 43.8 39.8 9.250 0.74
KAlSi3O8_glass 230.9 209.4 9.309 0.83
K2O.Al2O3.2SiO2 264.4 239.8 9.310 0.84
Mg3Al2Si3O12 359.2 325.5 9.395 0.89
KAlSiO4 132.2 119.8 9.423 0.84
KAl[SO4]2.3H2O 347.0 314.3 9.423 1.11
K2O.Al2O3.4SiO2 363.1 328.6 9.495 0.83
Ca2Mg5Si8O24H2 716.1 648.0 9.502 0.88
Na2B6O10 269.3 243.7 9.524 0.99
NaB3O5 134.7 121.8 9.524 0.99
KMg3[AlSi3O10][OH]2 393.2 355.7 9.552 0.94
MnSO4.4H2O 282.2 255.2 9.552 1.27
CaO.Al2O3 132.5 119.8 9.574 0.84
KAlSi2O6 181.5 164.1 9.583 0.83
Cu3P 97.2 87.8 9.628 0.44
NiCO3 78.6 86.2 9.630 0.66
ZnO.Cr2O3 130.7 143.4 9.651 0.56
NiSO4.H2O 151.0 136.4 9.680 0.87
SiO2 49.3 44.5 9.707 0.82
MgSO4 106.2 95.8 9.759 0.88
Fe[OH]3 112.5 101.5 9.817 1.05
Mg2Al3[AlSi5O18]H2O 554.5 500.0 9.831 0.92
ZnO.Al2O3 132.6 119.4 9.984 0.72
NaCa2Mg4Al3Si6O24H2 752.1 676.7 10.029 0.90
NaAlO2 81.8 73.5 10.051 1.00
Na2O.Al2O3 163.5 147.1 10.061 1.00
3MgO.[2SiO2].2H2O 304.7 273.9 10.093 1.10
Mg48Si34O85[OH]62 4929.5 4431.4 10.104 1.09
Na3PO4 167.0 150.0 10.133 1.02
NiSO4.4H2O 281.9 253.1 10.217 1.24
Mn2B 63.7 57.2 10.227 0.53
CuSO4.H2O 149.4 134.0 10.285 0.84
3CaO.[Al2O3].3SiO2 360.5 323.1 10.354 0.80
MnO.Fe2O3 135.1 149.2 10.416 0.59
MgSO4.4H2O 280.8 251.2 10.534 1.46
CaHPO4 122.3 109.4 10.544 0.90
Na2O.Cr2O3 161.6 178.8 10.616 0.76
Fe2MnO4 135.1 149.5 10.660 0.59
KAl[SO4]2 216.1 192.9 10.711 0.84
Fe2[SO4]3 293.9 262.4 10.737 0.74
NaAlSi3O8 229.7 204.8 10.835 0.88
Na2O.Al2O3.6SiO2 459.5 409.7 10.836 0.88
Al2[SO4]3.6H2O 554.2 494.1 10.841 1.23
Na2SO4.7H2O 443.1 395.0 10.849 1.65
NaAlSi3O8_alpha 229.7 204.8 10.850 0.88
Na2Mg3Al2Si8O22[OH]2 720.5 641.6 10.947 0.92
NaBH4 97.2 86.4 11.026 2.57
Mg3[PO4]2 239.7 213.1 11.094 0.91
K2O.4SiO2 270.5 300.7 11.161 0.81
KAl2[AlSi3O10][OH]2 367.0 325.9 11.198 0.92
NiSO4.6H2O 369.2 327.9 11.204 1.40
2CaO.Fe2O3 174.1 193.6 11.223 0.64
Al2[SO4]3 292.3 259.4 11.270 0.85
Fe2O3 94.1 104.8 11.299 0.59
Ca[H2PO4]2.H2O 291.9 258.8 11.342 1.16
CuO.Fe2O3 133.2 148.4 11.356 0.56
Al4B2O9 251.2 222.6 11.364 0.92
Na2O.Al2O3.4SiO2 360.8 319.8 11.380 0.89
NaAlSi2O6 180.4 159.9 11.380 0.89
KClO3 90.2 100.5 11.399 0.74
Al4Mg2Si5O18 510.9 452.3 11.460 0.87
Zn[OH]2 83.7 74.1 11.488 0.84
FeS2 70.3 62.2 11.516 0.59
Na2O.Al2O3.2SiO2 262.2 231.6 11.649 0.92
NaAlSiO4 131.1 115.8 11.649 0.92
NiSO4.7H2O 412.9 364.6 11.693 1.47
Na2HPO4 153.3 135.3 11.733 1.08
Co2P 73.4 64.8 11.736 0.49
KCl 46.2 51.7 11.877 0.62
Fe7S8 356.2 398.6 11.880 0.55
CaO.2Al2O3 225.1 198.2 11.943 0.87
NaCl 45.1 50.5 11.947 0.77
Al2Si4O10[OH]2 333.5 293.7 11.948 0.93
CaAl4Si2O10[OH]2 367.4 323.1 12.050 0.92
7MgO.8SiO2.H2O 715.3 629.0 12.061 0.92
AlCl3.6H2O 337.0 296.2 12.092 1.40
KAlSi3O8 230.9 202.9 12.110 0.83
CaMgSi2O6 178.2 156.6 12.118 0.82
NiFe2Cl8 211.8 237.5 12.129 0.47
CaO.Cr2O3 130.7 146.7 12.242 0.63
K2O.Fe2O3 167.4 187.9 12.247 0.66
MgO.Al2O3 132.1 115.9 12.250 0.93
Fe3O4 133.9 150.3 12.294 0.58
AgO 40.1 45.0 12.383 0.32
CaO.MgO.2SiO2 178.2 156.1 12.386 0.82
NaClO3 89.1 100.2 12.451 0.84
MgCl2.H2O 102.4 115.3 12.522 0.90
NaAlCO3[OH]2 163.3 142.6 12.661 1.13
Ni3Sn4 186.2 162.5 12.714 0.29
AlPO4 106.8 93.0 12.899 0.88
NaAl2[AlSi3O10][OH]2 365.9 318.5 12.967 0.96
CuSO4.5H2O 323.9 281.2 13.196 1.30
NiO.Cr2O3 131.4 148.8 13.216 0.58
KAl3Si3O10[OH]2 367.0 318.3 13.292 0.92
CuSO4.3H2O 236.6 205.0 13.372 1.11
KH 43.8 37.9 13.403 1.09
3Al2O3.2SiO2 376.3 325.4 13.509 0.88
Ni3P 102.1 87.8 14.007 0.49
Mg3Si4O10[OH]2 359.7 308.9 14.121 0.95
Al2O3.SiO2 141.9 121.6 14.263 0.88
CoO.Fe2O3 133.6 152.8 14.399 0.57
MgSiO3 88.9 101.7 14.407 0.89
SnO 41.7 47.7 14.549 0.31
CaO.Fe2O3 134.1 153.6 14.562 0.62
NaH 42.6 36.4 14.630 1.78
Al18B4O33 965.1 823.2 14.703 0.91
Ni2P 76.0 64.8 14.742 0.51
KAl3[OH]6[SO4]2 439.6 372.6 15.227 1.06
P2O5 121.0 102.4 15.398 0.85
AuCl 42.2 48.7 15.405 0.18
Mg5Al2Si3O10[OH]8 612.9 517.3 15.611 1.10
Na2H2P2O7 235.6 198.2 15.904 1.06
Na3AlCl6 210.4 244.4 16.150 0.68
SiI4 128.9 108.0 16.167 0.24
NaH2PO4 139.6 116.9 16.312 1.16
Fe[OH]2 83.4 97.0 16.377 0.93
AgClO3 86.3 100.5 16.413 0.45
K3AlCl6 213.8 249.0 16.481 0.60
Al2O3 92.5 77.2 16.534 0.91
AlP 48.1 40.1 16.714 0.83
Co[OH]2 83.1 97.1 16.792 0.89
K3Al2Cl9 288.9 337.4 16.795 0.59
[Al2O3].2SiO2 191.2 224.1 17.206 0.86
Mg3P2 122.4 101.0 17.435 0.91
K2O.Al2O3.6SiO2 461.7 380.9 17.502 0.83
KAlSi3O8_adular 230.9 190.5 17.502 0.83
Ni3Sn2 132.2 108.7 17.826 0.32
Al2O3.3H2O 223.5 183.5 17.899 1.43
NiO.Fe2O3 134.9 159.2 17.993 0.58
SiS2 65.2 77.5 18.866 0.71
NiCl2 60.0 71.7 19.507 0.46
Au2O3 94.6 114.0 20.540 0.21
ZnCl2 59.3 71.5 20.561 0.43
CoO.Cr2O3 130.1 157.2 20.811 0.57
Ca2MgSi2O7 218.2 172.7 20.851 0.80
MgCl2 58.8 71.2 21.024 0.62
MnCl2 60.2 73.0 21.177 0.48
MgCl 41.8 50.7 21.231 0.70
AlCl3 75.1 91.2 21.366 0.56
Fe2Al4Si5O18 511.2 401.3 21.492 0.79
AgClO2 71.7 87.3 21.830 0.41
Al2O3.H2O 136.2 106.3 21.973 1.14
Ca3MgSi2O8 258.2 199.2 22.821 0.79
Ni3C 86.8 106.7 22.882 0.46
CaCl2 59.2 72.8 23.018 0.53
CuCl2 58.3 71.9 23.196 0.43
CrCl3 74.2 91.8 23.762 0.47
Na2C2 73.4 91.0 24.016 1.05
CrCl2 57.2 71.2 24.364 0.47
AgCl 42.3 52.7 24.432 0.30
CaH2 54.3 41.0 24.479 1.29
AuCl3 76.1 94.8 24.552 0.25
ICl 44.2 55.3 25.179 0.27
NaO2 57.5 72.1 25.481 1.05
FeO 39.7 49.9 25.618 0.55
CuCl 41.4 52.0 25.665 0.42
CaO.[Al2O3].[2SiO2].2H2O 318.5 233.0 26.821 1.01
FeCl3 75.9 96.6 27.326 0.47
Fe2O3.H2O 137.8 175.7 27.538 0.78
Fe3C 83.7 107.1 27.946 0.47
SnCl2 60.9 78.0 28.197 0.32
CaO.2SiO2.[2H2O] 225.9 162.1 28.252 1.06
NiSO4 107.4 138.0 28.540 0.69
KAlCl4 121.3 156.5 28.978 0.58
NaAlCl4 120.2 155.0 28.988 0.63
FeCl2.4H2O 233.5 301.2 28.992 1.17
FeCl2 59.0 76.3 29.468 0.47
CaI 52.5 36.5 30.468 0.31
AuCl2 59.2 77.3 30.559 0.22
PCl5 108.6 142.5 31.259 0.52
Cr2O3 90.7 119.8 32.116 0.60
H2SO4.[3H2O] 241.2 318.9 32.242 1.59
NaC2H3O2 118.0 79.9 32.280 1.44
MnCl3 77.2 103.2 33.761 0.48
CoCl2 58.7 78.5 33.763 0.45
H2SO4.[4H2O] 284.8 382.2 34.188 1.67
MgH2 53.9 35.3 34.433 2.05
Co3S4 164.8 221.6 34.467 0.54
Mn3B4 123.2 79.8 35.245 0.59
CaOCl2 73.9 100.3 35.737 0.58
FeOCl 56.7 77.0 35.903 0.53
Si2H6 126.9 79.0 37.758 2.04
CoO 39.5 54.9 39.182 0.53
AlH3 67.7 40.3 40.479 2.26
C2Cl6 118.7 171.5 44.481 0.50
Cr[CO]6 162.5 240.2 47.781 0.74
PCl4 91.6 138.1 50.698 0.53
CaO2 54.6 82.8 51.593 0.76
CuH3 67.9 30.3 55.390 1.02
Fe2S3 117.9 47.8 59.468 0.57
Na2C2O4 132.0 34.0 74.248 0.99
2CaO.[Al2O3].8SiO2 567.1 97.7 82.781 0.82
CrO2 52.7 99.7 89.232 0.63
Ca3Al2Si3O12 360.5 1369.4 279.898 0.80
Ca2Al2SiO7 221.8 851.4 283.839 0.81
MgCO3.3H2O 208.4 -3689.2 1870.492 1.51 |
The relative error between the Kopp's law estimate and the default database is less than 7% for more than half of the data set, and only greater than 25% for 10% of the set. In fact, the largest error here (MgCO3.3H2O) has a negative Cp when calculated from the current default data, and some of the other very large errors look like the default Cp may be defective. It can be seen that testing via Kopp's Law is a good sanity check for any solid species. This approach also provides a method for estimating Cp when data is otherwise unavailable.
Note that the default SysCAD database continuously updated and is based on a range of open literature sources. As with all SysCAD default values, these are intended as a starting point for users, and we provide no guarantees for the veracity of the data.
The last column of the table shows the specific heat capacity calculated from the Kopp's law estimate (molar Cp divided by molecular weight). Across the data set, the values range from 0.17 to 2.57 kJ/kg.K. When no Cp data is supplied for a species in the Species Database, SysCAD uses a value of 1.0 kJ/kg.K which appears to be a reasonable estimate. (For more information see Species Table - Specific Heat (Cp).)
Given the 25 kJ/kmol.K observation of the Dulong-Petit Law, it is noted that, when applying this estimation method with Kopp's law, if the average atomic weight of elements in the compound is about 25 kg/kmol, then the Cp would be about 1.0 kJ/kg.K. As such, the deviation from 1.0 kJ/kg.K is related to the average atomic weight of the elements in the species. For species with heavier atoms such as AuSn, the calculated value is lower at 0.17, while species with lighter atoms such as NaBH4 is higher at 2.57. Both of these extreme examples however still give a good correlation with their respective values calculated from the default database.
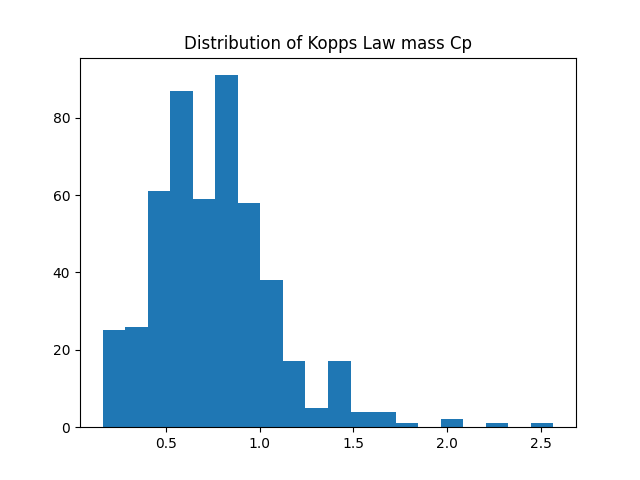
Plotting the distribution of all Kopp's law calculated specific heat capacities from this data set shows a mean value of ~0.75:
Conclusion
Leveraging the Kopp-Neumann Law, you can estimate the heat capacity of any solid species. This can be useful for identification of faulty data or to estimate a value when no other information is available. SysCAD uses 1.0 kJ/kg.K as a default for solid species, which, while a reasonable estimate, is dependent on the atomic weights of the involved elements. Therefore, the Kopp's law estimate may be a better option than the default in some cases.
It is important to keep in mind that Kopp's law provides an estimate only, so should only be used as a fall-back for minor species in a project. The heat capacities of major components should be based on verified thermodynamic data and lab measurements.
From Build 139.33288, the Kopp's law specific or molar heat capacity is shown alongside the calculated value for solid species on the Thermo1 tab of the Species Properties ($SDB) access window (Species > View Properties).
References
- (14 April 2023) "Dulong–Petit law", Wikipedia, Wikimedia Foundation, https://en.wikipedia.org/wiki/Dulong-Petit_law
- J Leitner, et al. (2003) "Estimation of heat capacities of solid mixed oxides", Thermochimica Acta, 395 27-46
First Posted: 13 July 2023
Reference Build: 139.33127
For questions or feedback, please contact us at [email protected].