Potash Solubility
Navigation: Models ➔ Potash Models ➔ Potash Species Model ➔ Potash Solubility
| Potash Species Model | Potash Solubility | Potash Properties Utility | Potash Evaporator |
|---|
Related Links: Species Models, Evaluation Blocks, Solubility, Plant Model - Soluble Tab
Overview
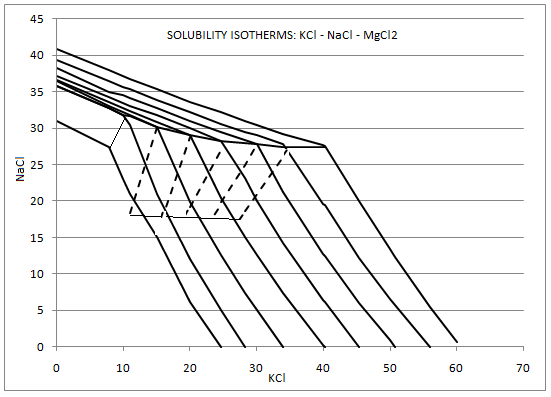
The solubility of KCl in Potash facilities is a function of temperature and is also influenced by the presence of other species in solution, most importantly NaCl and MgCl2. The curve for KCl solubility as a single species is not valid when other species are present, in this case the saturated value for KCl is lower than the saturated for the solution where only KCl is present.
Similarly, the saturated value for NaCl is lower in solutions that contain KCl and other species.
Ternary Saturation Diagrams using PHREEQC |
MgCl2 is often present in reasonably high concentrations in potash solutions, so this has a noticeable effect on the saturated values of KCl and NaCl. Other species that may also affect the saturation values are CaSO4, CaCl2 and LiCl. SysCAD has functionality that allows these species to be included in the calculation of KCl and NaCl solubilities.
Therefore, tables of saturated values for KCl and NaCl where other species are present have been included in SysCAD and are available in the Potash add-on.
SysCAD will automatically calculate the Heat of Reaction (HOR) for the dissolution or precipitation reactions for KCl and NaCl based on the thermodynamic data in the Species database for these species.
Implementation in SysCAD
- Users must have the Potash Add-on to use the Potash Solubility.
- Potash Solubility can not be enabled Globally in Build139 or later.
- Selectively applying solubility in unit models - The user may selectively enable solubility in units with Evaluation Blocks (EB) such as ties, tanks, pipes, etc.
- Solubility Display in Units - Information specific to the Potash Solubility is described in this section. The general solubility information shown in the access window of pipes, tanks, etc. is described in General Solubility Information in Units and Pipes.
- Enabling High MgCl2 region - 12 - 38g/100g - The region of low MgCl2 concentration, 0 to 12g/100g water, is based on data from literature1. The high MgCl2 concentration region has been interpolated from a small number of scattered data points. This region can be enabled in the Plant Model, but must be used with caution.
- The PHREEQC data set has been added in Build 139, user can select this data set from the Plant Model - Soluble Tab. For this DataSet, the maximum MgCl2 effect is 35g/100g. The imposed maximum is so that the entire solubility system is in a region where MgCl2 is below the saturation point.
Theory
KCl - NaCl Solubility
The dissolution of the following species produces ions in solution:
- [math]\ce{ KCl }[/math] → [math]\ce{ K+ }[/math] and [math]\ce{ Cl- }[/math];
- [math]\ce{ NaCl }[/math] → [math]\ce{ Na+ }[/math] and [math]\ce{ Cl- }[/math];
- [math]\ce{ MgCl2 }[/math] → [math]\ce{ Mg^2+ }[/math] and [math]\ce{ 2 Cl- }[/math].
- [math]\ce{ CaSO4 }[/math] → [math]\ce{ Ca^2+ }[/math] and [math]\ce{ SO4^2- }[/math];
- [math]\ce{ CaCl2 }[/math] → [math]\ce{ Ca^2+ }[/math] and [math]\ce{ 2 Cl- }[/math];
- [math]\ce{ LiCl }[/math] → [math]\ce{ Li+ }[/math] and [math]\ce{ Cl- }[/math].
Notes:
- In SysCAD we represent these ions as the species, for instance KCl(aq), NaCl(aq) and MgCl2(aq), with the understanding that these species do not actually exist as species, but rather as ions.
- If more than a single species exists in solution, the solubility of each will be affected by the other due to the interactions between the ions.
- This interaction can be described using the common ion effect at low concentrations of the species.
- However, as the concentrations of the species increase, this theory no longer correctly describes the solubility.
- The saturated values of KCl(aq) and NaCl(aq) in solutions containing either KCl and NaCl or KCl, NaCl have been obtained from values available in published data. (references 1 and 2).
- If any of the other optional species, MgCl2, CaSO4, CaCl2 or LiCl, are also present in the solution then they will depress the solubility of the KCl and NaCl. In SysCAD these other species are all combined into a single value and represented as 'MgCl2 Equivalent'. This value is then used to determine the depression of the solubility of the KCl and NaCl.
- The data is usually displayed in a graph with the form as shown below (ref. 1):
MgCl2 Equivalent
The presence of other species in solution will depress the solubility, and hence the saturation, values of KCl and NaCl. To determine the amount of depression, all of the species may be combined into a single 'MgCl2 Equivalent' value using the following formula:
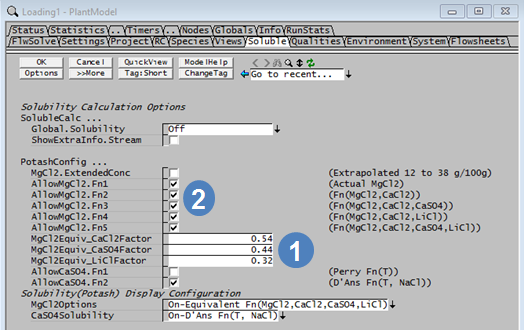
MgCl2 Equivalent = MgCl2 + a*CaCl2 + b*CaSO4 + c*LiCl where a, b and c are user defined factors that are set in the Plant Model access window.
The single MgCl2 Equivalent value is then used to determine the depression of solubility of the KCl and NaCl.
Notes:
- These species are all optional and will only be used by SysCAD if they exist in the project.
- The user defines the values of a, b and c on the Potash tab of the Plant Model access window. These constants can only be defined for species that exist in the actual project. For example, if the project configuration does not contain LiCl, then the field for LiCl will not be visible.
- The user may elect to ignore the effect of any of these species by either:
CaSO4 Solubility
The aqueous form of calcium sulphate, CaSO4(aq), is often present in solutions in Potash facilities. The precipitation / dissolution reaction is:
- CaSO4(aq) + H2O(l) <=> CaSO4.2H2O(s)
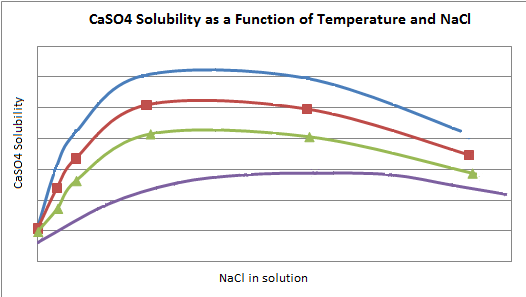
The solubility of CaSO4(aq) is also affected by the presence of other ions in solution. In the Potash Solubility calculations the user may choose to determine the solubility of CaSO4(aq) in solution using either of the following methods:
- Use solubility data from Perry (ref. 3) - therefore ignore any other species in solution and use data for 'pure' CaSO4; or
- Use solubility data from d'Ans (Ref. 1), which includes the effect of NaCl in solution. See the graph below:
Assumptions
- MgCl2 is NEVER saturated. The presence of this species affects the saturation values of KCl and NaCl, but it does not reach saturation. The MgCl2 saturation value is obtained from the data in Perry. Carnallite is never formed.
- The solubility data for D'Ans DataSet for KCl and NaCl is valid for values of MgCl2 (or equivalent) to a maximum value of 12g/100g water.
- For D'Ans Data for KCl and NaCl solubility above 12g/100g MgCl2 has been interpolated from the low MgCl2 region and a small amount of data for this region. The values in the high MgCl2 region MUST be used with caution.
- The solubility data for PHREEQC DataSet for KCl and NaCl is valid for values of MgCl2 (or equivalent) to a maximum value of 35g/100g water.
References
- d'Ans J. 1952 Die Bedeutung von Van' Hoff's Arbeiten Uber Losungsgleich-gewichte. Zeitschr. Electrochem.
- Garrett D. Potash Deposits, Processing, Properties and Uses.Chapman & Hall 1996
- Perry's Chemical Engineers' Handbook, 6th Edition
- PHREEQC Pitzer.dat database, PHREEQC is a computer program that is designed to perform a wide variety of aqueous geochemical calculations. It is developed and maintained by the United States Geological Survey. (refer to https://www.usgs.gov/software/phreeqc-version-3 for further information)
Solubility Examples
The solubility values displayed in the table on the 'PC' tab will be a function of the amounts of KCl, NaCl and MgCl2 in solution. The table below shows the values that will be displayed based on the amount of KCl and NaCl in solution:
Conditions:
- Temperature = 80°C
- 0% MgCl2 (The presence of MgCl2 depresses the saturated values of KCl and NaCl).
- Values of KCl and NaCl are given in g/100g water and are shown with 3 significant figures.
| Example | Actual KCl | Actual NaCl | Saturated KCl | Saturated NaCl | Region | Comment |
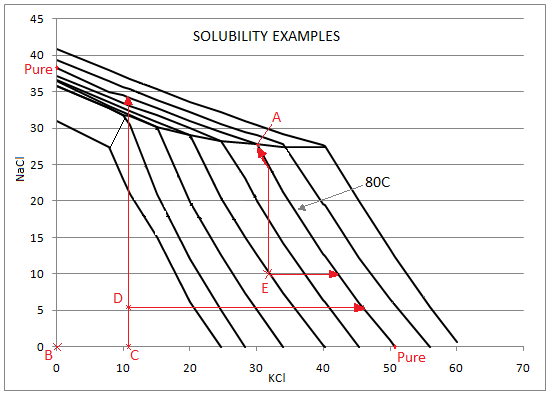
| A | 30.1 | 27.8 | 30.1 | 27.8 | KCl, NaCl: Sat | Both KCl AND NaCl are saturated - SysCAD displays the values for a solution saturated in both species - point A in the diagram below. |
| B | 0 | 0 | 50.9 (Pure) | 38.2 (Pure) | KCl, NaCl: None | There is neither KCl NOR NaCl in the stream - SysCAD displays the saturated values for each PURE component. |
| C | 11.1 | 0 | 50.9 (Pure) | 34.1 | KCl: Not Sat. NaCl: None | The stream contains ONLY KCl below saturation - SysCAD displays the saturated value of PURE KCl; and the saturated value of NaCl at the actual value of KCl in the stream (move up the vertical line from point C in the diagram below). |
| D | 11.1 | 5.8 | 45.6 | 34.1 | KCl, NaCl: Not Sat. | Both KCl AND NaCl are present in the stream below saturation - SysCAD displays the saturated value of KCl at the actual value of NaCl in the stream (move along the horizontal line from point D in the diagram below); and the saturated value of NaCl at the actual value of KCl in the stream (move up the vertical line from point D in the diagram below). |
| E | 33 | 10 | 42.1 | 27.8 | KCl, NaCl: Not Sat. | Both KCl AND NaCl are present in the stream below saturation - SysCAD displays the saturated value of KCl at the actual value of NaCl in the stream (move along the horizontal line from point E in the diagram below); and the saturated value of NaCl at the saturated value of KCl in the stream (In the diagram below move up the vertical line from point E, until it intersects with the KCl saturated line at 80C, then move to point A, where both species are saturated). |
These examples are displayed on the graph below:
Extra Info
The Extra Info table will display values for the solution at temperature with the following conditions:
- BothSat - Saturated in BOTH KCl and NaCl (Example A);
- SingleSat - Saturated values of each PURE component (Example B); and
- OneSat - Saturated value for each species at the ACTUAL or the SATURATED value of the other species, whichever is the lowest (Examples C, D and E).
To display the Extra Info table, go to View - Plant Model - Soluble and enable the ShowExtraInfo tick box for PC and/or Stream.
Data Sections
In this section we will only discuss the fields that are relevant for the Potash solubility.
- Solubility Display in Units - this describes the data fields specific to Potash solubility.
- Plant Model - Soluble Tab - this allows users to enable or disable Global solubility, change the level of display throughout the project, etc. NOTE: This is no longer used in Build 139 or later.
Solubility Display in Units
The fields on the PC tab of units and the Qo tab of pipes are described in this section.
Please see Phase Change (PC) for information on enabling Solubility in indivdual units.
| Tag (Long/Short) | Input / Calc | Description/Calculated Variables / Options |
| The following 2 fields are only available on the PC tab of units when Potash system is selected. They are NOT visible on the Qo tab of pipes. | ||
| Solubility (Potash) Calculation Configuration (Only visible if System = Potash) | ||
| MgCl2 Options The options that are available depend on the user selections in the Plant Model. |
Off | KCl(aq) and NaCl(aq) are the only aqueous species used when calculating the saturated values of KCl and NaCl from the D'Ans KCl-NaCl-MgCl2 graph. No other aqueous species are considered. |
| On | Include the actual concentration of MgCl2(aq) when calculating the saturated values of KCl(aq) and NaCl(aq) from the D'Ans KCl-NaCl-MgCl2 graph. CaCl2(aq) and CaSO4(aq) are ignored in the solubility calculations. | |
| Use Equivalent Fn (MgCl2, CaCl2) |
Use an 'equivalent' concentration of MgCl2(aq) when calculating the saturated values of KCl and NaCl from the D'Ans KCl-NaCl-MgCl2 graph. The Equivalent concentration is calculated from the concentrations of MgCl2(aq) and CaCl2(aq) in solution. CaSO4(aq) is ignored in the solubility calculations. | |
| Use Equivalent Fn (MgCl2, CaCl2, CaSO4) |
Use an 'equivalent' concentration of MgCl2(aq) when calculating the saturated values of KCl and NaCl from the D'Ans KCl-NaCl-MgCl2 graph. The Equivalent concentration is calculated from the concentrations of MgCl2(aq), CaCl2(aq) and CaSO4(aq) in solution. Note that both CaCl2(aq) and CaSO4(aq) are optional. | |
| The CaSO4 options are only available if both CaSO4(aq) AND CaSO4.2H2O(s) are included in the project. | ||
| CaSO4 Options | Off | CaSO4 solubility is not calculated or implemented. |
| Perry Fn(T) | Use the CaSO4 solubility as a function of Temperature, as defined in Perry, to calculate the saturated value of CaSO4 in solution. | |
| D'Ans Fn(T, NaCl) | Use the CaSO4 solubility as a function of Temperature and NaCl concentration, as defined by D'Ans, to calculate the saturated value of CaSO4 in solution. | |
| Solubility (Potash) Results (Only visible if System = Potash)
All of the following fields, up to the Summary table are visible in both the PC tab of units and the Qo tab of pipes. | ||
| T | Calc | The final temperature of the unit. |
| Region | Display | Please see Regions below for descriptions of the 15 different possible regions. |
| MgCl2.Actual | Calc | The actual amount of MgCl2 present. Displayed as mass MgCl2 / mass of water. |
| MgCl2.Equiv | Calc | The equivalent amount of MgCl2 present. Displayed as mass MgCl2 / mass of water. The method used to calculate the MgCl2 equivalent is described in the Theory section above. This field is only visible if MgCl2Options is NOT Off. |
| The following Fields and Tables are visible if ShowExtraInfo is enabled on the Soluble tab on the Plant Model access window (View - Plant Model - Soluble). | ||
| MgCl2.Sat | Calc | The saturated value of MgCl2 in solution. Displayed as mass MgCl2 / mass of water. (This value is calculated using the data from Perry.) |
| CaSO4.SingleSat | Calc | The Saturated value of CaSO4 as a single species solution. Displayed as mass CaSO4 / mass of water. (This value is calculated using the data from Perry.) |
| Saturated Data ignoring MgCl2. All values in the table below are calculated as if there was no MgCl2 in solution. Please see Solubility Examples above for each case. | ||
| BothSat | Calc | The saturated values of KCl and NaCl IF both species were saturated at the unit temperature. Displayed as mass species / mass of water. |
| OneSat | Calc | The saturated value of KCl with NaCl at either its actual or saturated value, whichever is the lowest. Similarly, the saturated value of NaCl with KCl at either its actual or saturated value, whichever is the lowest. Displayed as mass species / mass of water. |
| SingleSat | Calc | The saturated value of KCl with NO NaCl in solution (i.e. only KCl in solution). Similarly the saturated value of NaCl with NO KCl in solution (i.e. only NaCl in solution). Both values calculated at the unit temperature. Displayed as mass species / mass of water. |
| The following Table is only visible if MgCl2 Options is not OFF and ShowExtraInfo is enabled on the Soluble tab on the Plant Model access window (View - Plant Model - Soluble). | ||
| Saturated Data with MgCl2 = Eqv or Act. 'Eqv' (Equivalent) is displayed if the user has selected an MgCl2 equivalent, and 'Act' (Actual) will be displayed if the user has chosen to use the actual value of MgCl2. All values in the table below are calculated using the relevant value of MgCl2. | ||
| BothSat | Calc | The saturated values of KCl and NaCl IF both species were saturated at the unit temperature. Displayed as mass species / mass of water. |
| Saturated Data This Table is ALWAYS visible. | ||
| Act | Display | The actual values of the aqueous KCl, NaCl and CaSO4 in the solution. Displayed as mass species / mass of water. |
| Sat | Calc | The Saturated values for KCl, NaCl and CaSO4. These values are equal to the 'One Sat' values as described in the theory above, i.e. the saturated value for the species if the other species remained at its current value, or its saturated value at the current temperature, whichever is the lowest. Displayed as mass species / mass of water. |
| SatFrac | Calc | The fraction of saturation of KCl, NaCl and CaSO4 in the solution. These calculated as the Actual values / Sat values (where Sat value = One Sat value). |
| Summary The summary table is ALWAYS visible on the PC tab of units, for both Potash and standard solubility species. This table is NOT visible on the Qo tab of pipes. Please see Phase Change (PC) for a description of the fields in this table. | ||
Regions
The region in which the solution is located is displayed as a numerical value. The regions to which the values refer are described below:
| Region | KCl state | NaCl State |
| 0 | No KCl | No NaCl |
| 1 | No KCl | NaCl is present and is not saturated. |
| 2 | No KCl | NaCl is present and saturated. |
| 3 | No KCl | NaCl is present and supersaturated. |
| 4 | KCl is present and is not saturated. | No NaCl. |
| 5 | KCl is present and is not saturated. | NaCl is present and is not saturated. |
| 6 | KCl is present and is not saturated. | NaCl is present and is saturated. |
| 7 | KCl is present and is not saturated. | NaCl is present and is supersaturated. |
| 8 | KCl is present and is saturated. | No NaCl. |
| 9 | KCl is present and is saturated. | NaCl is present and is not saturated. |
| 10 | KCl is present and is saturated. | NaCl is present and is saturated. |
| 11 | KCl is present and is saturated. | NaCl is present and is supersaturated. |
| 12 | KCl is present and is supersaturated. | No NaCl. |
| 13 | KCl is present and is supersaturated. | NaCl is present and is not saturated. |
| 14 | KCl is present and is supersaturated. | NaCl is present and is saturated. |
| 15 | KCl is present and is supersaturated. | NaCl is present and is supersaturated. |
Plant Model - Soluble Tab
Only the fields that relate to Potash Solubility are shown here. Please see Plant model - Soluble Tab for information on the generic fields.
| Tag (Long/Short) | Input / Calc | Description/Calculated Variables / Options |
| Solubility Display Options | ||
| Potash.Show | Tickbox | Display the solubility values calculated using the Potash methodology. |
| Show Extra Info.PC | Tickbox | Show additional information related to solubility in all units that have Solubility enabled. |
| Show Extra Info.Stream | Tickbox | Show additional information related to solubility in all pipes. |
| PotashConfig... | ||
| DataSet | D'Ans | Data set D'Ans is valid for temperature range from 0 to 140 °C. The maximum MgCl2 effect is 12g/100g or 38g/100g depending on MgCl2.ExtendedConc selection. |
| PHREEQC | Available in Build139 and later. Data set generated from PHREEQC version 3 using the Pitzer.dat database, valid for temperature range from -20 to 199 °C. The maximum MgCl2 effect is 35g/100g. Please be aware that this has only been validated against the D'Ans data, and thus, data beyond D'Ans data applicability has not been validated against experimental data. | |
| MgCl2.ExtendedConc | Tickbox | Only visible with the D'Ans data set. Allow the model to use the data extrapolated into the high MgCl2 region (12 to 38g/100g water). Please do be aware that this data is not based on validated literature references. |
| AllowMgCl2.Fn1 | Tickbox | Allow the user to choose the actual MgCl2 in solution to calculate the MgCl2 concentration when determining the KCl and NaCl saturation values. |
| AllowMgCl2.Fn2 | Tickbox | Allow the user to choose the equation containing only MgCl2 AND CaCl2 in solution to calculate the equivalent MgCl2 concentration when determining the KCl and NaCl saturation values. |
| AllowMgCl2.Fn3 | Tickbox | Allow the user to choose the equation containing MgCl2, CaCl2 AND CaSO4 in solution to calculate the equivalent MgCl2 concentration when determining the KCl and NaCl saturation values. (LiCl is NOT included) |
| AllowMgCl2.Fn4 | Tickbox | Allow the user to choose the equation containing MgCl2, CaCl2 and LiCl in solution to calculate the equivalent MgCl2 concentration when determining the KCl and NaCl saturation values. (CaSO4 is NOT included in the calculation) |
| AllowMgCl2.Fn5 | Tickbox | Allow the user to choose the equation containing MgCl2, CaCl2, CaSO4 and LiCl in solution to calculate the equivalent MgCl2 concentration when determining the KCl and NaCl saturation values. |
| The following fields allow the user to set the factors that will be used when calculating the MgCl2 Equivalent. These factors can be obtained from plant or laboratory data. | ||
| MgCl2Equiv_CaCl2Factor | Input | The factor to be used for the CaCl2 term in the MgCl2 Equivalent equation. |
| MgCl2Equiv_CaSO4Factor | Input | The factor to be used for the CaSO4 term in the MgCl2 Equivalent equation. |
| MgCl2Equiv_LiClFactor | Input | The factor to be used for the LiCl term in the MgCl2 Equivalent equation. |
| The following tick boxes determine which solubility curves for CaSO4 will be available in the project. | ||
| AllowCaSO4.Fn1 | Tickbox | Allow the user to choose the CaSO4 curves from Perry3 to calculate the CaSO4 saturation values. |
| AllowCaSO4.Fn2 | Tickbox | Allow the user to choose the CaSO4 curves from D'Ans1 to calculate the CaSO4 saturation values. |
| Solubility (Potash) Display Configuration The options visible here will depend on the user selections above. | ||
| MgCl2 Options | Off | MgCl2 is not used when calculating the saturated values of KCl and NaCl from the D'Ans KCl-NaCl-MgCl2 graph. |
| On - Actual MgCl2 | Use the actual concentration of MgCl2 to calculate the saturated values of KCl and NaCl from the D'Ans KCl-NaCl-MgCl2 graph. | |
| On - Equivalent Fn (MgCl2, CaCl2) |
Use an 'equivalent' concentration of MgCl2 when calculating the saturated values of KCl and NaCl from the D'Ans KCl-NaCl-MgCl2 graph. The Equivalent concentration is calculated from the concentrations of MgCl2 and CaCl2 in solution. | |
| On - Equivalent Fn (MgCl2, CaCl2, CaSO4) |
Use an 'equivalent' concentration of MgCl2 when calculating the saturated values of KCl and NaCl from the D'Ans KCl-NaCl-MgCl2 graph. The Equivalent concentration is calculated from the concentrations of MgCl2, CaCl2 and CaSO4 in solution. | |
| On - Equivalent Fn (MgCl2, CaCl2, LiCl) |
Use an 'equivalent' concentration of MgCl2 when calculating the saturated values of KCl and NaCl from the D'Ans KCl-NaCl-MgCl2 graph. The Equivalent concentration is calculated from the concentrations of MgCl2, CaCl2 and LiCl in solution. | |
| On - Equivalent Fn (MgCl2, CaCl2, CaSO4, LiCl) |
Use an 'equivalent' concentration of MgCl2 when calculating the saturated values of KCl and NaCl from the D'Ans KCl-NaCl-MgCl2 graph. The Equivalent concentration is calculated from the concentrations of MgCl2, CaCl2, CaSO4 and LiCl in solution. | |
| CaSO4 Options | Off | CaSO4 solubility is not calculated or implemented. |
| On - Perry Fn(T) | Use the CaSO4 solubility as a function of Temperature, as defined in Perry, to calculate the saturated value of CaSO4 in solution. | |
| On - D'Ans Fn(T, NaCl) | Use the CaSO4 solubility as a function of Temperature and NaCl concentration, as defined by D'Ans, to calculate the saturated value of CaSO4 in solution. | |