Potash Species Model
Navigation: Models ➔ Potash Models ➔ Potash Species Model
| Potash Species Model | Potash Solubility | Potash Properties Utility | Potash Evaporator |
|---|
Related Links: Species Models
General Description
The Potash species model may be used to calculate certain properties of aqueous liquids within a MOP (Muriate of Potash) project. Density, Heat Capacity (and hence Enthalpy) and Viscosity may be calculated using the equations defined by in papers by Laliberté et al1,2,3. The Boiling Point Elevation (BPE) may be calculated using the Fabuss Korosi equation.
The Laliberté and Fabuss Korosi equations used in the species model are given in the Model Theory section below.
Notes:
- The Laliberté and/or Fabuss Korosi equations may be selected in the Plant Model, as described below.
- If the Laliberté species model is selected, only AQUEOUS species are considered when calculating liquid properties. If any of the following species are present they will be used in the calculations:
- KCl(aq), NaCl(aq), MgCl2(aq), CaCl2(aq), LiCl(aq), CaSO4(aq), NaBr(aq) and KBr(aq).
- All other aqueous species are IGNORED.
- All of the properties that are not explicitly calculated by this model are calculated using the Standard Species Model.
- The Four Stage Crystallisation Example and Three Stage Evaporator Example, which are distributed with SysCAD in the Examples Folder, demonstrate the use of the Potash species model in SysCAD projects.
Configuring Potash Species Model
In order for the Potash Species Model to be available in a project, it must included in the configuration file of the project.
Insert into Configuration file
Sort either by DLL or Group.
| DLL: | Potash.dll |
→ | Species Models | → | Potash | |
| or | Group: | Potash |
→ | Species Models | → | Potash |
See Model Selection for more information on adding models to the configuration file.
Model Theory
Liquid Density Calculations
The Laliberté solution density is calculated using the water density, pw and the solutes apparent density using following equation:
- [math]\displaystyle{ \mathbf{\mathit{p_m=\cfrac{1}{\frac{m_w}{p_w}+\sum{\cfrac{m_i}{p_{app,i}}}}}} }[/math]
- The Apparent density of each solute in solution is calculated from:
- [math]\displaystyle{ p_{app,i}=\cfrac{ c_0(1-m_w)+c_1 }{ 1-m_w + c_2 + c_3 T }e^{10^{-6}( T+c_4 )^2 } }[/math]
- Where:
mw = mass fraction of water mi = mass fraction of solute species i pw = density of water (at stream temperature and pressure), kg/m3 papp,i = solute i apparent density, kg/m3 pm = solution density, kg/m3 T = Temperature in °C c0 to c4 = dimensionless empirical constants for each solute species.
- Notes:
- The constants for most of the aqueous species are valid for temperatures between 0 and approximately 100°C.
- If the unit temperature is outside of the species temperature range, then SysCAD will use the values at the temperature limit.
- If the species Mass Fraction in a unit exceeds the maximum mass fraction, then SysCAD will continue to use the Laliberté equation, but will log a warning that the values are questionable.
- The constants for CaSO4(aq) are only valid for a single temperature, 25°C, and hence these values are suspect.
- Water density is calculated in SysCAD as described here: Water and Steam Properties.
- Solid density is calculated using the Standard species model method - Density Calculations using the Standard Species Model
Enthalpy Calculations
- With the Lalilberte model the user has a choice of Enthalpy models:
- The Laliberté model will calculate the Enthalpy as a full integral of Cp with respect to Temperature, i.e:
- [math]\displaystyle{ \Delta H = \int\limits_{T_1}^{T_2}Cp dT\, }[/math]
- The Laliberté Low model with calculate Enthalpy as
- [math]\displaystyle{ \Delta H = Cp * (T_2 - T_1) }[/math].
- This will be less accurate, but faster computationally.
- The Laliberté model will calculate the Enthalpy as a full integral of Cp with respect to Temperature, i.e:
- Note: Both methods will calculate Cp using the equations show below.
Heat Capacity Calculations
Liquid Heat Capacity
- With the Lalilberté model the liquid specific heat is calculated using the water specific heat, Cpw and the solutes heat capacity using following equation:
- [math]\displaystyle{ \mathbf{\mathit{Cp_m = m_w Cp_w + \sum{m_i Cp_i}}} }[/math]
- The heat capacity of each solute in solution is calculated from:
- [math]\displaystyle{ Cp_i= a_1e^{\alpha}+a_5(1-m_w)^{a_6} }[/math]
- Where
- [math]\displaystyle{ \alpha= a_2 T +a_3e^{T/100}+a_4(1-m_w) }[/math]
mw = mass fraction of water mi = mass fraction of solute species i Cpw = Heat capacity of water (at stream temperature and pressure), kJ/kg.K Cpi = Heat capacity of solute i, kJ/kg.K Cpm = solution Heat capacity, kJ/kg.K T = Temperature in °C a1 to a6 = dimensionless empirical constants for each solute species.
- Notes:
- The constants for most of the aqueous species are valid for temperatures between approximately 5 and 1200C.
- If the unit temperature is outside of the species temperature range, then SysCAD will use the values at the temperature limit.
- The constants for CaSO4(aq) are only valid for a single temperature, 250C, and hence these values are suspect.
- Water heat capacity is calculated in SysCAD as described here: Water and Steam Properties.
- Care needs to be taken with the coefficient values because of the single and double exponential terms: the constant [math]\displaystyle{ a_2 }[/math] would be expected to be very small or negative:
- [math]\displaystyle{ C_p \approx a_1\exp(a_2 T +a_3e^{T/100}) }[/math]
Solids and Vapours Heat Capacity
- Solids Cp (Cps) and Vapours Cp (Cpv) are calculated from Cp values as given in the species database, please see Specific Heat values (Cp) Calculations using the Standard Species Model.
Stream Heat Capacity
- [math]\displaystyle{ \mathbf{\mathit{Cp=\cfrac{SolidsMass*Cp_s+LiquidsMass*Cp_L+VapoursMass*Cp_V}{SolidsMass+LiquidsMass+VapoursMass}}} }[/math]
Viscosity
- With the Laliberté method, the liquid viscosity is calculated using the water viscosity, vw and the solutes viscosity using following equation:
- [math]\displaystyle{ \mathbf{\mathit{\ln{n_m} = m_w * \ln{n_w} + \sum{m_i * \ln{n_i}}}} }[/math]
- The viscosity for each solute is defined by:
- [math]\displaystyle{ \mathbf{\mathit{\ln{n_i} = \cfrac{v_1(1-m_w)^{v_2}+v_3}{(v_4*T+1)(v_5(1-m_w)^{v_6}+1)}}} }[/math]
nm = Solution Viscosity, mPa.s nw = Viscosity of water, mPa.s ni = Viscosity of solute i, mPa.s mw = mass fraction of water mi = mass fraction of solute i T = Temperature in °C v1 to v6 = dimensionless empirical constants.
- Notes:
- The constants for most of the aqueous species are valid for temperatures between approximately 5 and 1200C.
- If the unit temperature is outside of the species temperature range, then SysCAD will use the values at the temperature limit.
- CaSO4(aq) does not have any data for viscosity.
Boiling Point Elevation (BPE)
- Using the Fabuss Korosi method, the Boiling Point Elevation (BPE) of a brine is calculated by first determining the vapour pressure lowering of a solution containing KCl and NaCl relative to pure water. The activity coefficient is determined by a semi-empirical correlation.4,5
- [math]\displaystyle{ \mathbf{\mathit{k = \cfrac{p^0 - p}{mp^0}}} \quad }[/math] and [math]\displaystyle{ \quad \mathbf{\mathit{k = a + bu^{0.5}}} }[/math]
- where:
- k - Relative molar vapour pressure depression for a species
- m - molality of the solution
- p - vapour pressure of the solution
- p0 - vapour pressure of water at the given conditions
- u - Species ionic strength
- a and b - Temperature dependant constants, calculated as follows:
- [math]\displaystyle{ \mathbf{\mathit{a = a_1 + a_2t + a_3t^2}} }[/math]
- [math]\displaystyle{ \mathbf{\mathit{b = b_1 + b_2t + b_3t^2}} }[/math]
- t - Temperature
- The constants in the above equations, a1, a2, a3, b1, b2 and b3 are empirical values determined by experimentation.
- where:
References
- Laliberté M. and Cooper W.E. Model for Calculating the Density of Aqueous Electrolyte Solutions J. Chem. Eng. Data 2004, 49.
- Laliberté M. Model for Calculating the Viscosity of Aqueous Solutions J. Chem. Eng. Data 2007, 52.
- Laliberté M. A Model for Calculating the Heat Capacity of Aqueous Solutions, with Updated Density and Viscosity Data J. Chem. Eng. Data 2009, 54.
- Fabuss, B. M., Korosi, A. (1966). Vapour Pressures of Binary Aqueous Solutions of NaCl, KCl, Na2SO4 and MgSO4 at Concentrations and Temperatures of Interest in Desalination Processes. Desalination, 1, 139-148.
- Fabuss, B. M., Korosi, A. (1966). Vapour Pressures of Ternary Aqueous Solutions of NaCl, KCl, Na2SO4 and MgSO4 at Concentrations and Temperatures of Interest in Desalination Processes. Desalination, 1, 149-155.
Required Chemical Compounds
The following species are used by the Potash set of models and are required in the species database and configuration file. Some of the species are optional, but if they are present in the database, their effects will be included in the correlation calculations.
| Phase | Species Formula | Species Name | Required / Optional |
| VAPOUR | H2O(g) | Water Vapour | Required |
| AQUEOUS SPECIES | H2O(l) | Water | Required |
| KCl(aq) | Aqueous Potassium Chloride | Required | |
| NaCl(aq) | Aqueous Sodium Chloride | Required | |
| MgCl2(aq) | Aqueous Magnesium Chloride | Optional | |
| CaCl2(aq) | Aqueous Calcium Chloride | Optional | |
| LiCl(aq) | Aqueous Lithium Chloride | Optional | |
| CaSO4(aq) | Aqueous Calcium Sulphate | Optional | |
| KBr(aq) | Aqueous Potassium Bromide | Optional | |
| NaBr(aq) | Aqueous Sodium Bromide | Optional | |
| SOLIDS | KCl(s) | Solid Potassium Chloride | Required |
| NaCl(s) | Solid Sodium Chloride | Required | |
| MgCl2(s) | Solid Magnesium Chloride | Optional | |
| CaCl2(s) | Solid Calcium Chloride | Optional | |
| CaSO4.2H2O(s) | Gypsum | Optional | |
| CaSO4(s) | Anhydrous Gypsum | Optional |
Selecting different methods for Property Calculations
If the Potash species model is selected, the user can choose to use Laliberté or the Standard method to calculate Liquid Density, Specific Heat (Cp) or Viscosity.
The user may also choose either the Fabuss Korosi or the standard method to calculate the Boiling Point Elevation.
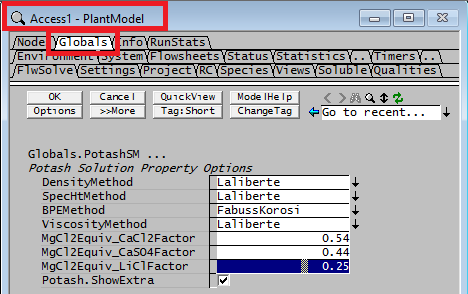
These selections are applied globally, so you cannot use different methods in different areas in the same project. The method is selected globally from the View - PlantModel access window Globals Tab as illustrated below.
Potash Liquor Calculator
When defining a Potash Liquor stream, it is possible to have SysCAD calculate the feed composition based on user defined potash species concentrations.
This is done in the Feeder unit - but only in a 'True' feeder, i.e. not connected to a sink on a different flowhseet:
- First turn on the Calculator, by ticking the 'Calculator' checkbox located on the FeederSink Tab,
- Then go to the newly created tab called "Calc", and define the required composition.
This is shown in the images below:
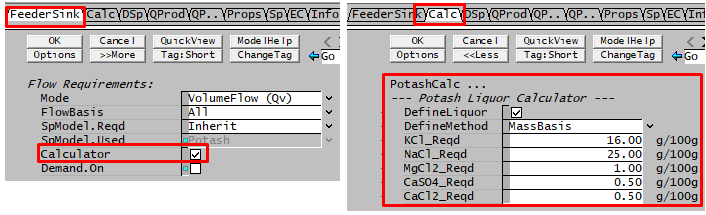
- For more information on the feed calculator variables, please see Potash Calculator Configuration Data.
The calculated feed composition will be set into the DSp tab. The variables set by the calculator are indicated by the yellow background.
NOTES:
- If the calculator is enabled, the user may NOT manually change the feed composition on the DSp tab. All changes must be made on the Calc tab.
- The user may enable the calculator once to get an idea of the values, and then turn the calculator off to manually change the DSp tab values, if required.
- If the feeder is connected to a sink on another flowsheet, the calculated values are ignored.
Data Sections
- The specific Potash data will be displayed on the 'Props' tab for the Qi and Qo pages of the Pipe access window, and under the Content page for units.
- If the user has selected the Laliberté methods for calculating Density, Heat Capacity and Viscosity, then these will be used and displayed throughout the project.
- Only the data that is calculated using the Potash equations is shown below. The other data is discussed in the SysCAD Model help - Pipe Section.
Feeder Configuration Data
| Tag (Long/Short) | Input / Calc | Description |
| Potash Liquor Calculator | ||
| DefineLiquor | Check Box | If this box is checked, the Feeder will calculate the fractional make-up of the feed stream, based on the variables supplied below. |
| Define Method | Mass Basis | The user may enter the required aqueous species as g/100 water. |
| Concentration | The user may enter the required aqueous species as g/L of solution. | |
| Saturation | NOT YET IMPLEMENTED! The user may enter the required aqueous species as % saturated. | |
| KCl_Reqd | Input | The required KCl value |
| NaCl_Reqd | Input | The required NaCl value |
| MgCl2_Reqd | Input | The required MgCl2 value (if MgCl2 is in the project) |
| CaSO4_Reqd | Input | The required CaSO4 value (if CaSO4 is in the project) |
| CaCl2_Reqd | Input | The required CaCl2 value (if CaCl2 is in the project) |
Potash Data
This data will be displayed on the 'Props' tab of pipes and Contents. Note: The term 'Salts' refers to all Chloride salts, i.e. KCl, NaCl, LiCl, MgCl2 and CaCl2.
| Tag (Long/Short) | Input or Calc | Description |
| Potash (Liquid Phase) Values | ||
| AqSaltsQm | Calc | The mass flow of aqueous salts in the stream. |
| AqSaltsFrac | Calc | The mass fraction of aqueous salts in the liquid. |
| AqK2OEquivQm | Calc | The mass flow of aqueous KCl expressed as K2O. |
| AqK2OEquivFrac | Calc | The mass fraction of aqueous KCl in the liquid, expressed as K2O. |
| AqMgCl2EquivQm | Calc | The mass flow of aqueous MgCl2, CaCl2 and CaSO4, expressed as MgCl2 equivalent. |
| AqMgCl2EquivFrac | Calc | The mass fraction of aqueous MgCl2, CaCl2 and CaSO4 in liquid, expressed as MgCl2 equivalent. |
| Potash (Solid Phase) Values | ||
| SolSaltsQm | Calc | The mass flow of solid, or crystal, salts in the stream. |
| SolSaltsFrac | Calc | The mass fraction of solid, or crystal, salts in the solid. |
| SolK2OEquivQm | Calc | The mass flow of solid, or crystal, KCl expressed as K2O. |
| SolK2OEquivFrac | Calc | The mass fraction of solid, or crystal, KCl in the solid, expressed as K2O. |
| Potash Properties These values are calculated using the methods chosen by the user in the 'Plant Model'. | ||
| LRho | Calc | The liquor density. |
| SatRho | Calc | The density of a solution saturated in BOTH KCl and NaCl at the stream temperature. |
| LmsCp@T | Calc | The Heat Capacity of the solution at the stream temperature. |
| LmsHs@T | Calc | The sensible enthalpy of the solution at the stream temperature. |
| LViscosity | Calc | The Viscosity of the solution at the stream temperature. |
| Potash Specific Properties (Laliberté-Cooper) These values are calculated using the Laliberté method. They are only used in the project if the user has chosen method = Laliberté in the Plant Model. (In which case these values will be the same as the ones in the above section). These values are only displayed if the user ticks the 'Potash.showExtra' box in Potash Solution Property Options in the Plant Model. | ||
| Potash.LRho | Calc | The liquor density at temperature using Laliberté method. |
| Potash.LmsCp@T | Calc | The Heat Capacity of the solution at the stream temperature using Laliberté method. |
| Potash.LmsHs@T | Calc | The sensible enthalpy of the solution at the stream temperature using Laliberté method. |
| Potash.LmsHs@T_Low | Calc | The sensible enthalpy of the solution at the stream temperature calculated using the low fidelity Laliberté method. |
| Potash.LViscosity | Calc | The Viscosity of the solution at the stream temperature using Laliberté method. |
| OutOfRange.Density | Calc | This will display any species in the stream that are outside of the range of the Laliberté constants for density. If all species are in range this field will be blank. |
| OutOfRange.Cp | Calc | This will display any species in the stream that are outside of the range of the Laliberté constants for Cp. If all species are in range this field will be blank. |
| OutOfRange.Viscosity | Calc | This will display any species in the stream that are outside of the range of the Laliberté constants for viscosity. If all species are in range this field will be blank. |
| Potash Specific Properties (Fabuss-Korosi) The Boiling Point Elevation is calculated using the Fabuss Korosi method. This is only used in the project if the user has chosen method = Fabuss Korosi in the Plant Model. (In which case this value will be the same as the one in the above section). This value is only displayed if the user ticks the 'Potash.showExtra' box in Potash Solution Property Options in the Plant Model. | ||
| Potash.BPE | Calc | The liquor Boiling Point Elevation at temperature using the Fabuss Korosi method. |