Calculating Energy change around a unit
Navigation: Models ➔ Model Examples ➔ Calculating Energy change around a unit
Calculating Energy change around a unit
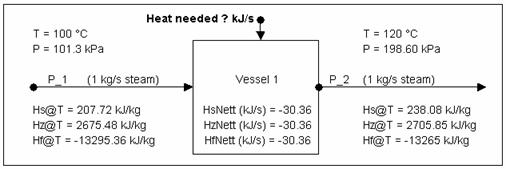
Calculating Energy change around a unit without phase change
The above is a 'summary' of information you can obtain from SysCAD.
Now to check the numbers using the steam tables:
- At 100 °C @ sat. P, Hg = 2675.572 kJ/kg
- At 120 °C @ sat. P, Hg = 2705.9342 kJ/kg
Thus energy required to heat up the steam for 20 °C is:
1 kg/s * (2705.9342 - 2675.572) kJ/kg = 30.3622 kJ/s (answer = to that obtained from SysCAD.)
As demonstrated by the above example, you can use Hs (sensible heat), Hz (enthalpy) or Hf (heat of formation) values to work out the energy required to raise the stream by 20°C. It is whatever you are comfortable with.
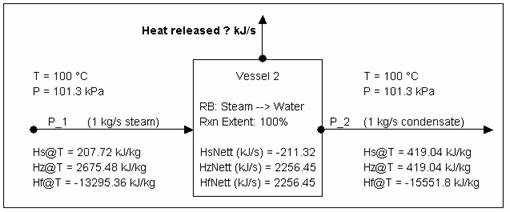
Calculating Energy change around a unit with phase change (via Reaction block)
Consider the above example but with all the steam condensing.
Due the presence of phase change, we need to get some additional information to check the energy balance. Since the above example is done via a reaction block, the following information is available:
- [email protected] (kJ/s) = -2256.92 = energy released
- [email protected] (kJ/s) = 244.97 *
- (* You need this value because all Hs calcs in SysCAD are referenced back to 0 °C.)
Energy balance using SysCAD numbers:
Using Hs: 207.72 kJ/kg * 1 kg/s + 211.32 kJ/s - 419.04 kJ/kg * 1 kg/s = 0 kJ/s
Using Hz: 2675.48 kJ/kg * 1 kg/s - 2256.45 kJ/s - 419.04 kJ/kg * 1 kg/s = 0 kJ/s
Using Hf: -13295.36 kJ/kg * 1 kg/s - 2256.45 kJ/s - (-15551.8 kJ/kg) * 1 kg/s = 0 kJ/s
Energy Balance check using Steam Table:
At 100 °C @ sat. P, Hg = 2675.572 kJ/kg, Hl = 419.0992 kJ/kg,
Thus the latent heat is: Hlg = 2675.573 - 419.0992 = 2256.4729 kJ/kg
Therefore energy released is 2256.4729 kJ/kg * 1kg/s = 2256.47 kJ/s (cf 2256.45 from SysCAD.)
NOTES on using Cps for energy balance checks
1) dH = mass flow * average Cp * dT can be generally applied to estimate the enthalpy change for liquids. Assuming Cp change is linear and pressure has little effect.
2) The above equation cannot be used for steam as Cp change is not linear and pressure does have huge effect. It is recommended to work out the enthalpy changes for steam using the Hz values (which are same as Hg or Hl values from the steam tables, alternatively, latent heat values can be used).
3) For more examples on energy balances around unit operations, please see the excel spreadsheets from sample projects provided. Sample energy balances can be found in sample projects:
Leach.spf - Leach.xls from the Tutorial projects.
NiCuDemo.spf - Ni Demo1.xls from Nickel demo projects.