Discussion: New Reaction Heat Features in Alumina Precipitation Models
Navigation: Product Blog ➔ Discussion Pages ➔ New Reaction Heat Features in Alumina Precipitation Models
Related Links: Precipitation3, Alumina 3 Bayer Species Model
Overview
In Build 139.30140, we have reworked the way that reaction heats are defined and used in the Alumina Precipitator model. For historical reasons, the overall energy balance for the model was originally based on a default or user-specified heat of reaction for Alumina. This option is still available, but now by default, the calculations will be based on heat of formation data (H25) in the SysCAD species database (SDB). Using these new calculation methods will require enthalpy data for all involved species. New options will allow additional specification of reaction heats for co-precipitation of Caustic (Bound/Occluded Soda) and Oxalate. The new options are available in both the Steady-State and Dynamic solvers. For simple precipitation reactions, which can be implemented as Reaction Blocks in a Tank model, the temperature change in the Precipitator model is identical to that in the Tank model.
Background
Dissolution and Precipitation of Alumina are key components in the Bayer Process for production of Alumina from Bauxite. These are described by the reaction:
- [math]\ce{ Al[OH]3(s) + NaOH <=> NaAl[OH]4 }[/math]
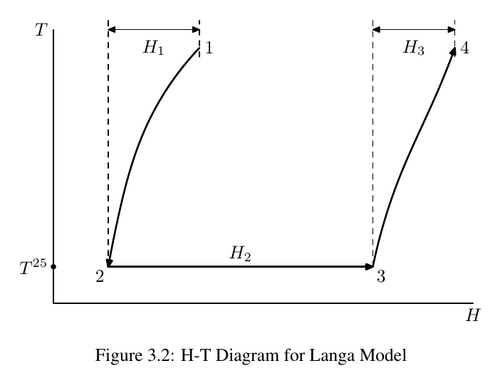
Dissolution is endothermic, requiring addition of heat, while precipitation is exothermic, releasing heat. Management of thermal issues surrounding the process is key to successful plant operation. Heat recovery in digestion is important for energy efficiency, while cooling in precipitation to maintain a temperature/supersaturation profile is necessary for product quality. Thus in modelling the Bayer process, having good data on the heat of reaction (HOR) for this reaction is critical. The basis for such calculation is described by Langa[1] and illustrated here by the dissolution reaction. (For precipitation, the calculations are identical, but reversed.)
Starting at point (1), we cool the liquor (and solid THA (trihydrate alumina)) at fixed composition to the reference temperature 25°C (2), add heat at isothermal condition while dissolving THA to point (3), then reheat the liquor and remaining THA to the original temperature (4). Because the liquor has different properties before and after dissolution of THA, the heats [math]\displaystyle{ H_1 }[/math] and [math]\displaystyle{ H_3 }[/math] are different. The heat [math]\displaystyle{ H_2 }[/math] is determined by H25 data for the individual species involved; in this case it will be:
- [math]\displaystyle{ H^{25}(\hbox{NaAl[OH]4}) - H^{25}(\hbox{NaOH}) - H^{25}(\hbox{Al[OH]3}) }[/math] J/mol
The overall heat of reaction at temperature [math]\displaystyle{ T }[/math] HOR@T is then:
- [math]\displaystyle{ -H_1+H_2+H_3 }[/math]
where [math]\displaystyle{ H_1 }[/math] and [math]\displaystyle{ H_3 }[/math] are calculated by integrating the specific heat:
- [math]\displaystyle{ \int_{25}^T C_p(T) dT }[/math]
Using this approach, Langa and others have calculated typical HOR values for dissolution and precipitation. SysCAD has a built-in HOR@0 default value of 252.3 kJ/kg of THA for dissolution. The HOR@T can be calculated by integration for any given liquor compositions. However, this value itself is calculated for typical liquor compositions and may not be accurate in other cases. Instead, we introduce a rigorous implementation of the Langa approach based on user H25 data, along with options to allow user-specified H25 values.
The existing energy balance attempted to avoid any reaction heat issues by requiring you to specify HOR in both the precipitator model and in general Reaction Blocks (RB).
To enforce the latter, H25 was often left out in the SDB, necessitating HOR override when setting up reactions manually. We now consider this to be a bad practice, and discourage its use in the future.
New Calculation Options
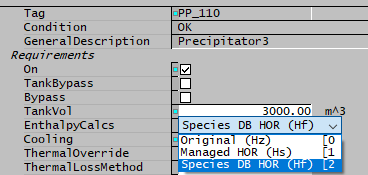
On the first Precipitator3 tab there is a new option dropdown list EnthalpyCalcs, containing:
- Original (Hz)
- Managed HOR (Hs)
- Species DB HOR (Hf)
The Original (Hz) option is for backwards-compatibility - existing projects will use this and continue to give the same results. However, there are some technical issues with this option and we strongly recommend you convert to using one of the new options options. Please note that this option is deprecated and will be removed in future releases.
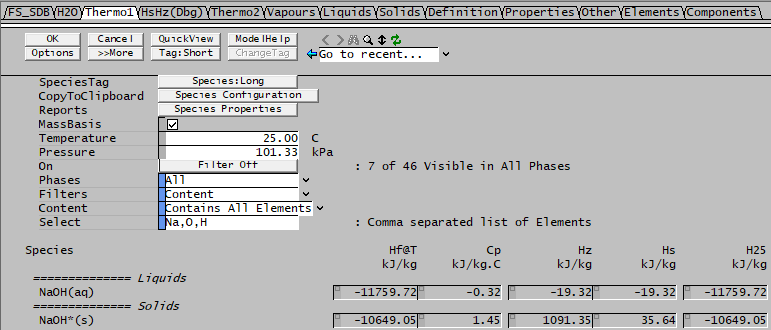
The problem with this option is that partial information in the SDB may affect the results, since it uses Hz for calculations, which accounts for heat of phase change for the species in the reaction. You can get different results depending on which components (phases) of the reacting species have a specified H25. To see what species have a well-defined H25, you can use the Species > View Properties menu and look in the Thermo1 tab. For example, for Soda co-precipitation [math]\ce{ NaOH(aq) -> NaOH\mbox{*}(s) }[/math] we have:
For this project, the H25 values are defined for the following species:
H25(NaOH(aq)) = -11759 kJ/kg H25(NaOH*(s)) = -10649 kJ/kg
So the HOR@25 is just -10649 - (-11759) = 1110 kJ/kg and this will be incorporated into the overall energy balance.
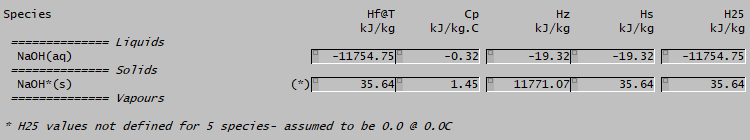
If you look at the distributed Alumina projects, you may see a different number:
The (*) indication here for bound soda (NaOH*(s)) indicates that H25 is not defined for this species. SysCAD then assign a Hf value of 0 at 0°C and calculates a H25 value by integrating the Cp equation (giving the value 35.64). In the energy balance, the HOR@25 used will then be 35 - (-11754) = 11719 kJ/kg, which is ten times as high as the value when both H25 values are defined!
The new options remove any ambiguity in the calculation, so are more rigorously based. You should review all of your alumina projects and add H25 values for the alumina species; you can then use the new options in your models.
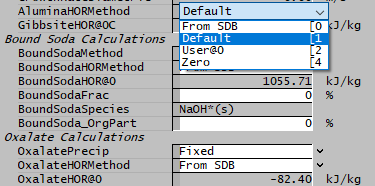
Managed HOR
The Managed HOR option allows you to specify the reaction heat for Alumina, Soda and Oxalate in a number of ways.
- From SDB - Calculate the value of HOR from species database (SDB)
- Default - Fixed default value
- User@0 - User-specified HOR@0
- Zero - HOR@0 = 0
The Default and User-Specified values are equivalent to the present options of allowing either a default or user-specified value. The additional options give more flexibility - you could specify a HOR for THA, and fall back on the SDB values for the other components.
Species DB HOR
The Species DB HOR option effectively calculates all the reaction heats from the H25 values in the SDB. In order to use this, you need to supply H25 values for (at minimum) the following species
| Al[OH]3(s) | Alumina Hydrate | -1293.13 kJ/mol |
| NaAl[OH]4(aq) | Soluble Aluminate | -1741.14 kJ/mol |
| NaOH(aq) | Free Caustic | -470.355 kJ/mol |
| NaOH*(s) | Bound Soda solids | -425.93 kJ/mol |
| Na2C2O4(s) | Oxalate | -1315.0 kJ/mol |
| Na2C2O4(aq) | Dissolved Oxalate | -1305.57 kJ/mol |
You can import these from any of the available databases or input the values manually. If any of these are missing, the model will disallow selection of the Species DB HOR.
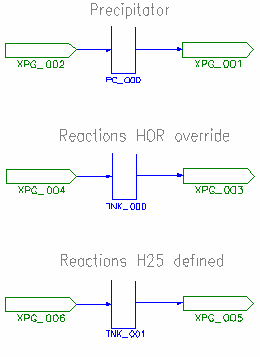
Equivalence to Tank Reaction Models
We can compare the results of the Precipitator models with simple Tank models by implementing reactions in the latter and controlling the extent to achieve the same precipitation rate.
- The Managed HOR models give the same final temperature as a tank RB with HOR override
- The Species DB HOR models give the same final temperature as a standard tank RB
In summary, these new options implement a more rigorous calculation of the precipitator energy balance and eliminate possible side-effects of undefined data. You can then use the alumina species in other reactions without having to specify HOR. For example, suppose we have subsequent calcination of THA via the reaction in a RB:
- [math]\ce{ 2Al[OH]3(s) -> Al2O3(s) + 3H2O(g) }[/math]
There is now no need to override the HOR in the RB, which would be the case if the H25 for [math]\ce{ Al[OH]3 }[/math] is undefined.
References
- "The Heat of Dissolution of Gibbsite at Bayer Digestion Temperatures", Langa, Light Metals 1985, 197-208
First Posted: 31 January 2022
Reference Build: 139.30140
For questions or feedback, please contact us at [email protected].